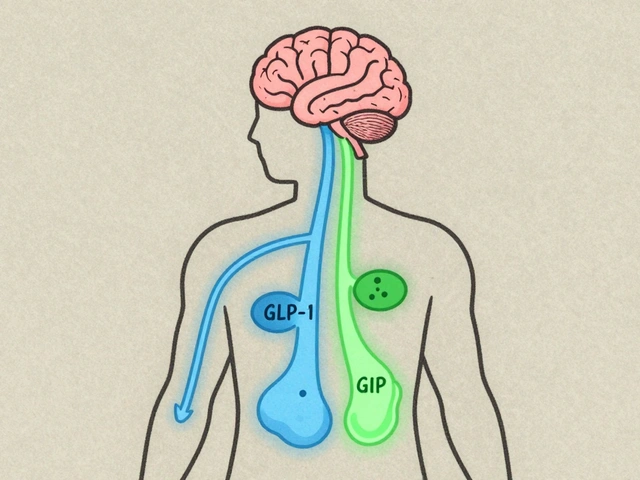
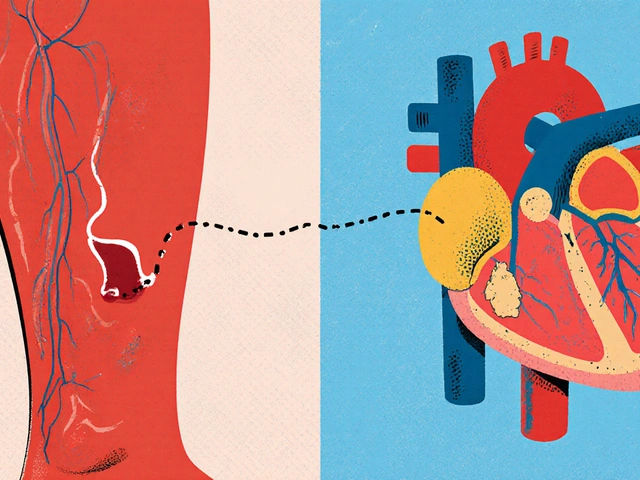
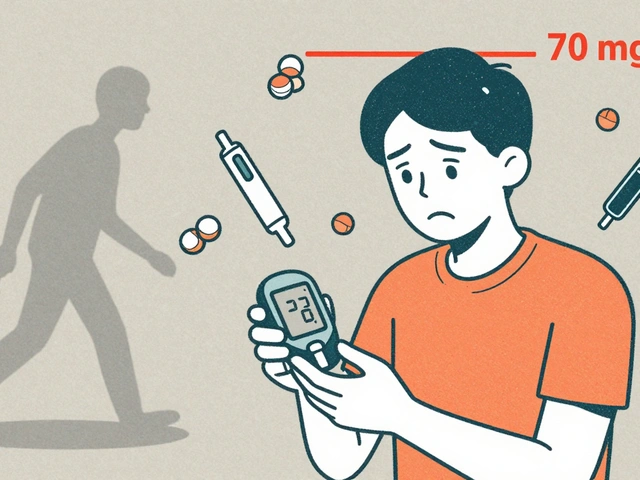
FDA Approved: What It Really Means for Your Medications
When you see FDA approved, a designation from the U.S. Food and Drug Administration that means a drug has been reviewed for safety, effectiveness, and manufacturing quality before being sold to the public. Also known as regulated pharmaceutical approval, it’s the baseline trust mark for every pill, injection, or patch you take. But here’s the thing: FDA approved doesn’t mean flawless. It means the benefits outweigh the risks—at least in the controlled conditions of clinical trials. Real-world use? That’s where things get messy. A drug can be FDA approved and still cause unexpected side effects, interact dangerously with other meds, or even have manufacturing flaws that slip through the cracks.
Behind every FDA approval is a long process: lab tests, animal studies, human trials, and factory inspections. The agency checks for consistent dosing, proper labeling, and clean production lines. But approval doesn’t guarantee every batch is perfect. That’s why you’ll find posts here about generic drug defects, common manufacturing issues like capping, contamination, or weight variation that can reduce effectiveness or cause harm, and why some people react differently to generics than brand-name versions. Culture, language on the label, even the color of the pill can affect whether someone takes it as prescribed. And while the FDA sets standards, enforcement isn’t always consistent—especially when supply chains are stretched thin or production moves overseas.
It’s also not just about new drugs. The FDA reviews changes to existing ones too—new dosing, new combinations, new delivery methods. That’s why you’ll see articles on drug shortages, how federal actions in 2025 are trying to fix broken supply lines with AI tracking and stockpiles of active ingredients, and why some medications vanish from shelves even if they’re FDA approved. The system is designed to protect you, but gaps exist in monitoring, economic incentives, and how quickly problems get fixed.
What you’ll find below isn’t a list of approved drugs. It’s a look at what happens after approval: how side effects show up in real people, how manufacturing flaws sneak in, how cultural beliefs shape trust, and how even approved meds can turn dangerous when mixed with other substances. You’ll learn why a statin taken at night or morning might not matter as much as sticking to the same time every day, why herbal teas can clash with blood thinners, and how a simple misstep in temperature control can ruin insulin before it even reaches your fridge. This isn’t about marketing claims. It’s about what you need to know to use your meds safely—no matter who approved them.
- Nov 27, 2025
- Posted by Cillian Osterfield
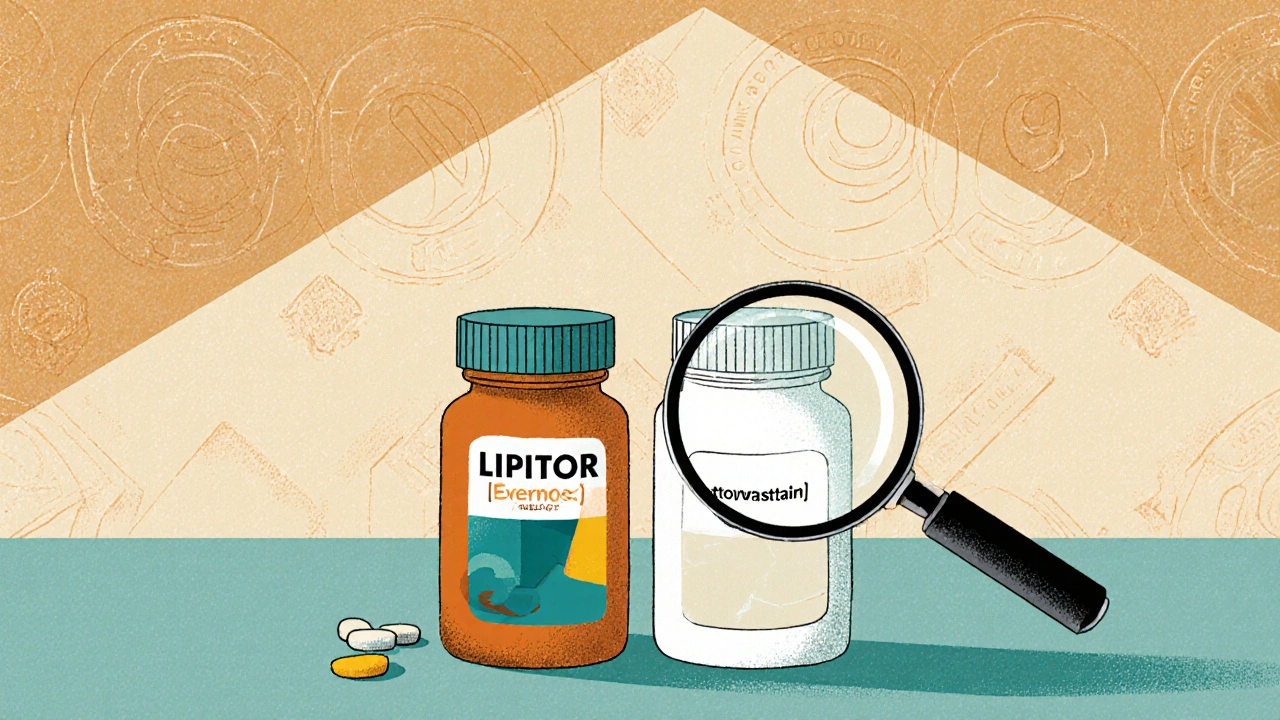
Authorized Generics vs Brand Drugs: What You Need to Know About Identical Medications
Authorized generics are identical to brand-name drugs in every way - same ingredients, same factory, same results. Learn how they work, why they cost less, and when they’re the smartest choice for your health.
Categories
- Health and Wellness (72)
- Medications (68)
- Health and Medicine (28)
- Pharmacy Services (12)
- Mental Health (9)
- Health and Career (2)
- Medical Research (2)
- Business and Finance (2)
- Health Information (2)
Latest Posts
©2026 heydoctor.su. All rights reserved