Ever opened your prescription bottle and seen a different name on the label - same pill, same size, same color - but no brand name you recognize? That’s not a mistake. It’s an authorized generic. And if you’re paying for medication out of pocket, it could save you serious money - without changing a single thing about the drug you’ve been taking.
They’re the exact same drug - just without the brand name
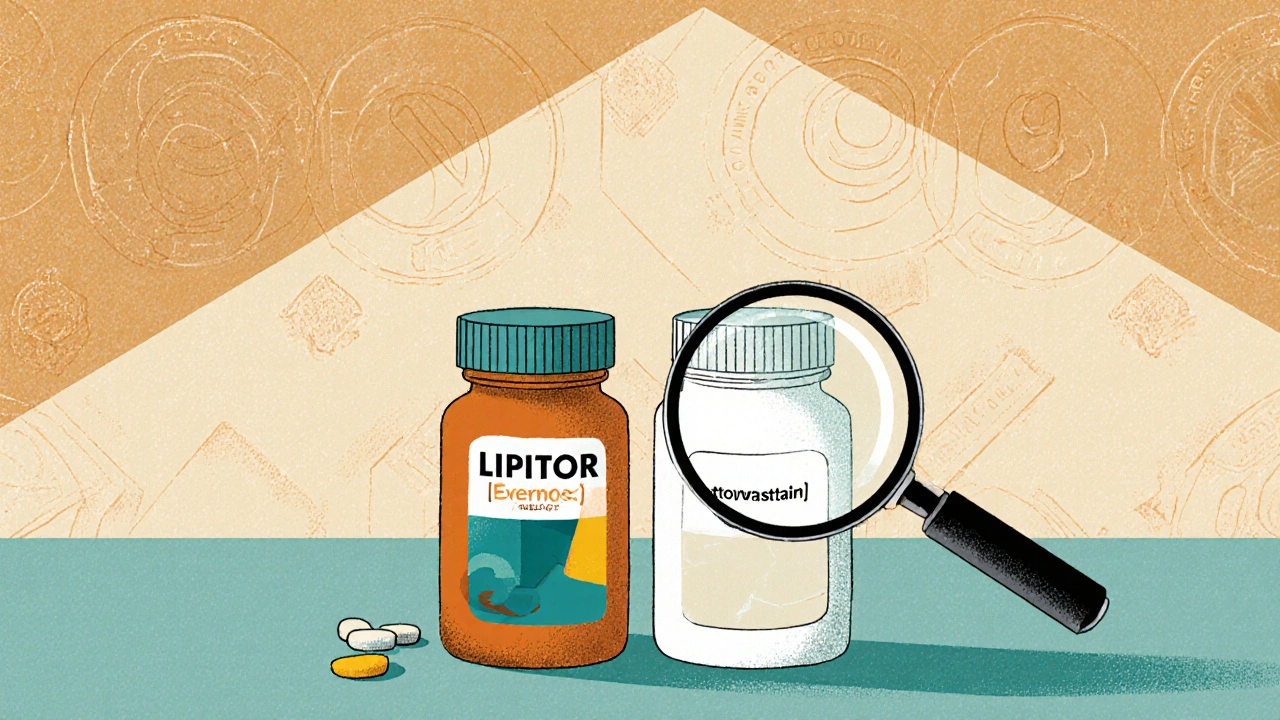
An authorized generic isn’t a copy. It’s not a knockoff. It’s the exact same pill your doctor prescribed, made in the same factory, with the same active ingredients, the same fillers, the same coating, and the same manufacturing process. The only difference? The label doesn’t say "Lipitor" or "Zoloft" - it just says "atorvastatin" or "sertraline."
The U.S. Food and Drug Administration (FDA) defines it clearly: an authorized generic is a brand-name drug sold without the brand name on the package. It’s made under the original drug’s New Drug Application (NDA), meaning it doesn’t go through the usual generic approval process. That’s why it’s chemically identical - down to the last inactive ingredient.
Traditional generics? They’re required to have the same active ingredient and work the same way, but they can use different fillers, dyes, or binders. For most people, that doesn’t matter. But for some - especially those with allergies, sensitivities, or conditions like celiac disease - those tiny differences can cause real problems. Authorized generics remove that uncertainty.
How they’re made: No extra approval, no guesswork
Here’s how it works: A company like Pfizer makes a drug called Lipitor. After the patent expires, another company can make a generic version of atorvastatin. But Pfizer can also choose to make its own generic version - same tablet, same packaging, just no "Lipitor" logo. That’s an authorized generic.
It doesn’t need a new FDA application. It doesn’t need bioequivalence studies. It’s already been approved. The FDA just needs to know the brand company is selling it under a different label. That’s why authorized generics show up in the FDA’s database - but not in the Orange Book, where traditional generics are listed with therapeutic equivalence ratings.
This means you’re getting the same product your doctor prescribed, but without the marketing costs, brand recognition premiums, or fancy packaging. It’s the same factory, same batch, same quality control - just sold cheaper.
Price difference: Not always what you expect
You might assume authorized generics cost the same as traditional generics. They don’t.
Traditional generics can be 80-85% cheaper than brand-name drugs. Authorized generics? Often only 15-30% cheaper. Why? Because the brand manufacturer controls the supply. They’re not trying to compete on price - they’re trying to compete on market share. By offering an authorized generic, they keep customers from switching to a third-party generic that might be even cheaper.
For example, if brand-name Zoloft costs $120 for a 30-day supply, the traditional generic might be $10. The authorized generic? Maybe $40. Still a savings - but not the deep discount you’d hope for.
That’s why some people get confused. They see a lower price and think, "This must be inferior." But no - it’s the same drug. The price gap isn’t about quality. It’s about business strategy.

Why do brand companies even make them?
It sounds counterintuitive: Why would a drugmaker undercut their own product? The answer is control.
When a patent expires, multiple generic companies can enter the market. Prices crash. Profits vanish. By launching their own authorized generic, the original brand company can:
- Keep some market share instead of losing it all
- Control the supply chain and quality
- Prevent a third-party generic from becoming the default choice
- Keep patients loyal to their brand’s formulation
This isn’t new. It’s been happening since the 1980s, after the Hatch-Waxman Act created the modern generic approval system. Now, over 150 authorized generics are on the market, covering everything from cholesterol meds to antidepressants to asthma inhalers.
Are they safer? For some people, yes
Most patients don’t notice any difference between generics and brand drugs. But for a small group - people with severe allergies, autoimmune conditions, or digestive sensitivities - inactive ingredients matter.
Traditional generics might use corn starch, lactose, or dyes that trigger reactions. Authorized generics use the same inactive ingredients as the brand. So if you’ve had a bad reaction to a generic before - even if it was "therapeutically equivalent" - switching to the authorized version might be the fix.
A 2018 study of over 5,000 patients found no major difference in hospital visits or medication adherence between those using authorized generics versus brand drugs. But the real win? Patients who switched from a traditional generic to an authorized one reported fewer side effects - especially those with known sensitivities.
The American Academy of Allergy, Asthma & Immunology recommends authorized generics for patients who’ve had reactions to traditional generics. Their position is simple: if your body reacts to fillers, don’t risk it. Use the version with the same ingredients you already tolerated.
What you’ll see at the pharmacy
When you pick up your prescription, the pharmacist might hand you a bottle labeled "sertraline" instead of "Zoloft." They’re allowed to substitute generics unless your doctor wrote "DAW" (Dispense As Written) on the script.
But here’s the catch: many pharmacists don’t explain the difference. Patients often assume "generic" means "cheap version," not "exact copy." That’s why about 30% of people question the switch - even when the drug is identical.
Ask your pharmacist: "Is this an authorized generic?" If they’re unsure, ask for the manufacturer’s name. If it’s the same company that makes the brand drug - like Pfizer, AstraZeneca, or Johnson & Johnson - then you’ve got the authorized version.
You can also check the FDA’s website for the latest list of authorized generics. It’s updated regularly. If the drug is on there, and the manufacturer matches the brand, you’re getting the real deal.
Insurance and coverage: It’s complicated
Insurance plans love generics. They’re cheaper. But with authorized generics, things get messy.
Some plans treat them like traditional generics - same copay. Others put them on the brand tier because they’re made by the brand company. That means you could pay more for an authorized generic than for a traditional one - even though they’re identical.
Always check your formulary. If your plan covers the brand drug, ask if the authorized generic is included. If it’s not, ask your doctor to write "DAW" on the prescription. That forces the pharmacy to give you the brand - but you’ll pay more.
Or, if you’re paying cash, compare prices. Sometimes the authorized generic is cheaper than the brand, even if your insurance doesn’t cover it. Use tools like GoodRx or SingleCare to compare cash prices across pharmacies.
Bottom line: Same drug. Lower cost. Fewer surprises.
Authorized generics aren’t a loophole. They’re not a trick. They’re a straightforward way to get the exact same medication you’ve been prescribed - without paying for branding.
They’re perfect if:
- You’ve had bad reactions to traditional generics
- You want consistency in how your body responds
- You’re paying out of pocket and want a middle-ground price
- You trust the brand’s formulation and want to avoid any risk
They’re not ideal if:
- You’re looking for the deepest discount - traditional generics are cheaper
- Your insurance won’t cover them and the cash price is too high
- You don’t care about inactive ingredients
Bottom line? If you’ve ever wondered whether generics are "good enough," authorized generics answer that question with a clear yes - they’re not just good enough. They’re identical.
Next time you get a prescription, ask: "Is there an authorized generic?" You might be surprised how easy it is to save money - without sacrificing anything.
Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made under the same FDA-approved New Drug Application (NDA) as the brand-name drug. They contain identical active and inactive ingredients, are produced in the same facility, and follow the same manufacturing standards. The FDA confirms they are therapeutically equivalent to the brand drug.
Why are authorized generics sometimes more expensive than regular generics?
Because they’re made by the original brand manufacturer, they don’t face the same price competition as traditional generics. Brand companies use authorized generics to retain market share, so they price them higher - often only 15-30% below the brand name, not the 80-85% discount seen with third-party generics.
Can I ask my pharmacist for an authorized generic specifically?
Yes. You can ask your pharmacist if an authorized generic is available for your medication. If they don’t know, ask for the manufacturer’s name. If it matches the brand-name maker - like Pfizer or Merck - then it’s an authorized generic. You can also check the FDA’s authorized generic list online.
Do authorized generics show up in the FDA’s Orange Book?
No. Authorized generics are not listed in the Orange Book because they’re sold under the brand’s original New Drug Application (NDA), not through the Abbreviated New Drug Application (ANDA) process used by traditional generics. You’ll need to check the FDA’s separate authorized generics list for this information.
Are authorized generics covered by insurance like regular generics?
Sometimes. Insurance plans vary. Some treat authorized generics like traditional generics and put them on the lowest copay tier. Others classify them as brand-name drugs because the manufacturer is the same. Always check your plan’s formulary or call your insurer to confirm coverage.






14 comments
Geethu E
I switched to an authorized generic for my antidepressant last year after my old generic gave me crazy stomach issues. Same pill, same results, no more bloating. Saved me like $50 a month too. Why didn’t anyone tell me this sooner?
jaya sreeraagam
Okay, let me just say this because I’ve been researching this for months and I’m obsessed with the science behind it-authorized generics are not just ‘the same drug,’ they’re literally the exact same batch of medication, just repackaged under a different label, and that’s why they’re so underutilized! Most people think generic = cheap knockoff, but no, it’s like buying the same Starbucks coffee but in a plain cup instead of the branded one-same beans, same barista, same temperature, just no logo. And if you have sensitivities to dyes or fillers? This is your golden ticket. The FDA doesn’t lie, and the manufacturers don’t mess with their own production lines unless they’re trying to keep you hooked. So stop assuming ‘generic’ means ‘inferior.’ It’s marketing, not medicine.
doug schlenker
My dad’s on a blood thinner and he had a bad reaction to the generic last year. We switched to the authorized version and he hasn’t had a single issue since. Honestly, if you’ve ever had weird side effects from a generic, this is worth asking for. Pharmacies don’t always tell you.
Nicola Mari
It’s pathetic how people treat medication like it’s a fashion choice. You’re not saving money by being ‘smart’-you’re risking your health with inferior formulations. If you want the real thing, pay for the brand. Don’t gamble with your body just to save $20.
Alexis Mendoza
So if it’s the same pill, why does it cost more than the other generic? That doesn’t make sense.
Chris Kahanic
Authorized generics are a fascinating case study in pharmaceutical economics. The brand manufacturer leverages its existing regulatory approval and production infrastructure to maintain market share post-patent, effectively creating a pseudo-monopoly on the low-cost segment. It’s not altruism-it’s strategic pricing. The consumer benefits from consistency, but not necessarily from price competition.
anant ram
Guys, seriously-ask for the authorized generic! I’ve been doing this for years, and it’s saved me so much money-and no more weird rashes from the other generics! The manufacturer name is the key! Always check it! Don’t just take what they give you! You have rights! Ask! Ask! Ask!
king tekken 6
lol so the big pharma companies are like ‘oh we’ll make our own generic so we can still make money’-classic. they’re not trying to help you, they’re just trying to keep you from going to a real cheap generic. they don’t care if you’re broke. they just want their slice of the pie. it’s all about control, man. the system is rigged.
DIVYA YADAV
This is why India is the pharmacy of the world. We make better generics than these American companies who charge $100 for a pill that costs 5 cents to produce. Why are we even talking about authorized generics? Just import real Indian generics-they’re cheaper, better tested, and not owned by some CEO in New Jersey who thinks you’re dumb enough to pay for a logo.
Kim Clapper
I find it deeply concerning that this post is framed as ‘empowering.’ It’s not. It’s a corporate strategy disguised as consumer advice. You’re being manipulated into choosing a slightly cheaper version of the same product, while the real solution-universal drug pricing and transparency-is ignored. This is not a win. It’s a distraction.
Bruce Hennen
Authorized generics are not identical. They’re manufactured under the same NDA, yes, but the packaging, labeling, and distribution channels are different. That alone introduces variability in stability and bioavailability. Don’t be fooled.
Jake Ruhl
so like… if the brand company makes the generic… does that mean they’re secretly the only ones who can make it? like… what if they just stop making the authorized version? then you’re stuck with the brand or the weird generic? it’s a trap. they’re playing chess while we’re playing checkers. i feel used.
Chuckie Parker
Just ask for the authorized one. If they don’t have it, ask for the brand. If they won’t give you either, get a new pharmacist. Simple.
George Hook
I’ve been on the same medication for 12 years. I started with the brand, switched to a generic that gave me migraines, then switched to the authorized version-and I haven’t had a single side effect since. The inactive ingredients matter more than people think. If you’ve ever had a bad reaction to a generic, this isn’t just a cost-saving tip-it’s a quality-of-life fix. I wish I’d known this five years ago.