When a brand-name drug’s patent runs out, prices don’t just drop-they collapse. But when exactly does that happen? And how many generic versions will flood the market? For pharmaceutical companies, getting this wrong can mean losing hundreds of millions in revenue overnight. For generic manufacturers, missing the window means wasting millions on failed development. Predicting generic entry isn’t guesswork-it’s a high-stakes science built on patents, regulations, and competitive strategy.
How the System Works: The Hatch-Waxman Act and ANDAs
The foundation of generic drug entry in the U.S. started in 1984 with the Hatch-Waxman Act. This law created a legal shortcut for generic companies to bring cheaper versions to market without repeating the expensive clinical trials that brand-name drugmakers had to do. Instead, they file an Abbreviated New Drug Application, or ANDA. The catch? They can’t submit it until the brand’s patent expires-or if they challenge the patent, they can file early but risk a lawsuit. The real game-changer? The 180-day exclusivity period for the first generic company to file an ANDA challenging a patent. That’s a huge incentive. If you’re first, you get to be the only generic on the market for six months before anyone else can enter. That’s when prices start to tumble. The brand might still be selling, but now you’ve got 80% of the market at 60% less cost. That’s why companies race to be first.What Actually Delays Generic Entry?
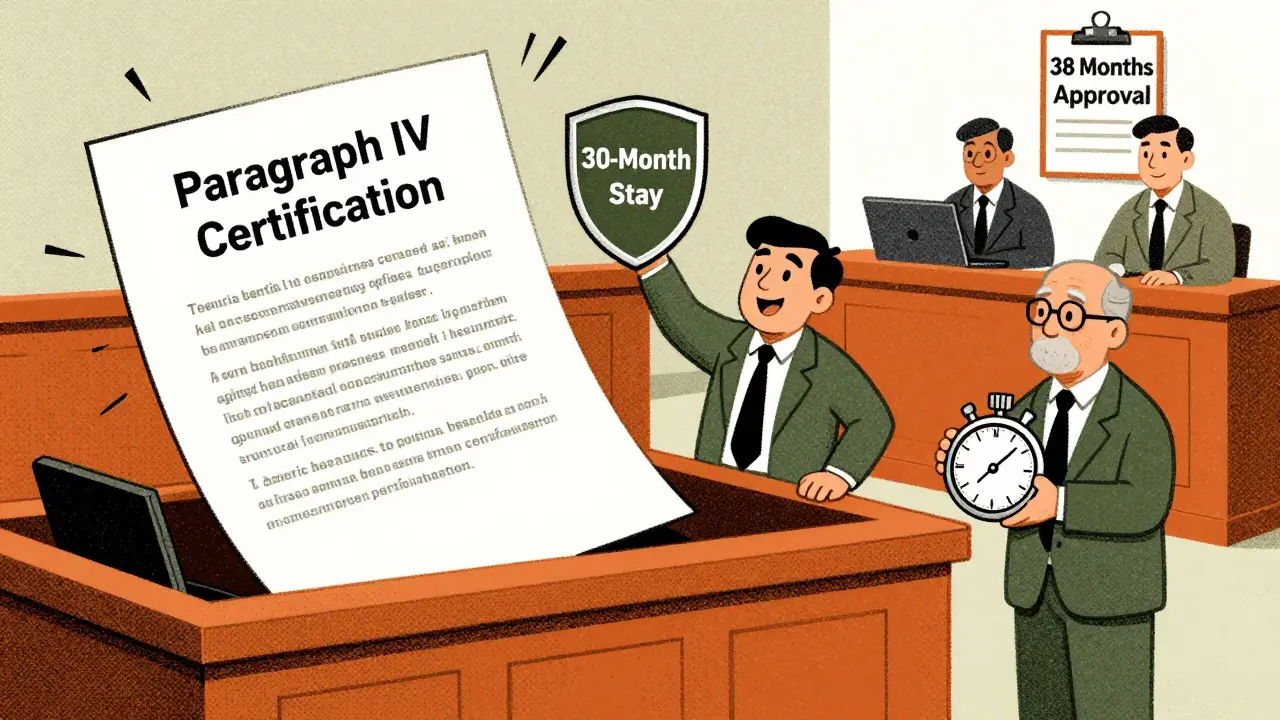
You’d think if a patent expires on January 1, 2026, generics arrive the next day. But that almost never happens. Here’s what really slows things down:- Patent litigation: If a generic company files a Paragraph IV certification (meaning they say the patent is invalid or won’t be infringed), the brand can sue. That automatically triggers a 30-month stay. On average, this delays entry by 18.7 months.
- Regulatory delays: The FDA takes about 38 months to approve an ANDA from submission to approval. If a company submits too early, they’re just waiting. If they submit too late, they miss the window.
- Product hopping: Some brands change the drug’s form-switching from a pill to a capsule, or adding a new delivery system-right before the patent expires. This tricks pharmacists and doctors into thinking it’s a new drug. In 63% of top-selling drugs, this tactic extends market control by 18 to 24 months.
- Authorized generics: Sometimes, the brand company itself launches a generic version under a different label. This happens in 41% of cases, but most forecasting models miss it entirely.
- REMS programs: Risk Evaluation and Mitigation Strategies, meant to control dangerous side effects, can block generic access if the brand refuses to share distribution systems. This delays entry by an average of 14.3 months.
How Accurate Are the Forecasts?
Simple models that just look at patent expiration dates? They’re wrong about half the time. R² values (a statistical measure of accuracy) hover around 0.45-meaning less than half the variation in timing is explained by the patent date alone. Advanced models use 40+ data points: patent litigation outcomes, FDA approval timelines, market size, therapeutic class, and even the number of patents clustered around the drug. The best models-like Evaluate Pharma’s J+D Forecasting-use machine learning to analyze 15 years of ANDA data. They’re hitting R² values of 0.82. That’s a 35% improvement over basic models. But even the best models struggle with:- Biologics: These are complex proteins, not simple chemicals. They’re not called generics-they’re biosimilars. Approval takes 12-18 months longer, and price drops are slower. Only 25-35% of the original price falls after three biosimilars enter, compared to 85% for small-molecule drugs.
- Complex generics: Inhalers, injectables, topical creams-these require specialized manufacturing. Approval times average 52 months, not 38. Forecasting these is like predicting a weather pattern with missing sensors.
- Pay-for-delay deals: Sometimes, the brand pays the generic company to delay entry. These secret settlements are hard to detect until they’re revealed in court.
Price Drops After Generic Entry
Once generics start rolling in, prices don’t just fall-they spiral:- First generic: Drops price by 39% below brand level.
- Second generic: Price falls another 15%, totaling 54% below brand.
- Fifth or sixth generic: Prices hit 85% below original brand price.
Who Uses These Forecasts-and Why?
Brand-name companies use them to plan. If they know a $1.2 billion drug will lose exclusivity in 14 months, they might:- Launch a new formulation (product hop)
- Start a patient transition program (like AbbVie did with Humira → Skyrizi)
- Adjust pricing or marketing budgets

What’s Changing in 2026?
The landscape is shifting fast:- Competitive Generic Therapy (CGT) pathway: The FDA now offers 180-day exclusivity to generics targeting drugs with little competition. This is new, and models are still learning how to predict it.
- AI models: By 2026, AI-driven tools will cut prediction errors from 11.4 months to under 7 months by reading court filings, FDA letters, and patent claims automatically.
- Medicare drug price negotiation: Starting in 2025, Medicare will negotiate prices for some high-cost drugs. That could reduce the price drop after generics enter by 15-20%-because the brand was already priced lower than expected.
- State substitution laws: California’s 2022 law made it harder for pharmacists to switch patients to generics without doctor approval. That slowed price declines by 8.2% compared to national models.
What You Need to Know
If you’re in pharma-whether you’re on the brand side or the generic side-here’s what matters:- Don’t rely on patent expiration dates alone. They’re just the starting line.
- Track Paragraph IV certifications. They’re the first real signal that a generic challenger is coming.
- Monitor FDA Orange Book updates weekly. That’s where patent and exclusivity data live.
- Watch for product hopping. If a brand suddenly changes the dosage form or adds a new delivery system, assume they’re trying to delay generics.
- Know your therapeutic class. Oncology drugs delay generic entry 32% longer than cardiovascular drugs.
How long does it take for a generic drug to hit the market after patent expiration?
It’s not instant. Even if a patent expires on January 1, 2026, the first generic might not launch until 12 to 24 months later. Delays come from patent lawsuits (which can add 18+ months), FDA approval timelines (average 38 months from submission), or strategic delays like product hopping. The fastest generics enter within 6 months if there’s no litigation and the ANDA was submitted early.
What’s the difference between a generic drug and a biosimilar?
Generic drugs are exact chemical copies of small-molecule drugs, like pills or tablets. Biosimilars are similar-but not identical-to complex biologic drugs made from living cells, like insulin or cancer antibodies. Biosimilars take longer to develop (12-18 months vs. 12-24 months for generics), cost more to produce, and face stricter approval rules. Price drops are also slower: biosimilars reduce costs by 25-35% after three competitors, while generics drop 85%.
Why do some generic drugs never come to market?
Three main reasons: patent litigation delays, complex manufacturing requirements (like inhalers or injectables), and lack of profit incentive. If a drug has low sales or high production costs, generic makers won’t risk the investment. Also, if the brand company launches its own generic (authorized generic), there’s no financial upside for others to enter.
Can a brand company legally delay generics?
Yes, but within legal limits. They can file lawsuits when a generic challenges their patent, triggering a 30-month stay. They can also change the drug’s form (product hopping), extend exclusivity with pediatric studies, or file citizen petitions to delay FDA approval. While some tactics are controversial, many are legal under current rules. The FTC has cracked down on pay-for-delay deals, but product hopping and patent thickets remain largely unchallenged.
How do I find out when a specific drug’s patent expires?
Check the FDA’s Orange Book, which lists patents and exclusivity periods for brand-name drugs. It’s updated weekly. You can also use commercial tools like Drug Patent Watch or Cortellis Generics Intelligence, which combine Orange Book data with litigation tracking and FDA approval timelines. For accurate forecasts, you need more than just the patent date-you need the full legal and regulatory context.