When a generic drug hits the market, you expect it to work just like the brand-name version. But how do regulators make sure it does? For simple pills, checking peak concentration (Cmax) and total exposure (AUC) used to be enough. But for complex drugs-like extended-release painkillers, abuse-deterrent opioids, or heart medications that need steady levels-those old metrics often miss the real story. That’s where partial AUC comes in.
Why Traditional Metrics Fall Short
Think of a drug’s journey through your body like a car trip. Total AUC tells you how far the car drove. Cmax tells you how fast it went at its peak. But what if the car needs to accelerate quickly at the start to avoid an accident-or slow down gradually to stay on the road? That’s the problem with older bioequivalence tests. They don’t care about when the drug is absorbed, just how much and how high. For drugs that release slowly over hours, or that have multiple release phases (like a fast-acting layer plus a slow one), two products can have the same Cmax and total AUC but behave completely differently in your body. One might spike too fast and cause side effects. Another might take too long to kick in and leave you untreated. Traditional metrics can’t catch that. And in some cases, they’ve let unsafe generics through. A 2014 study in the European Journal of Pharmaceutical Sciences found that 20% of generic drugs that passed old bioequivalence tests failed when partial AUC was added. When researchers looked at both fasting and fed conditions together, failure rates jumped to 40%. That’s not a small glitch-it’s a gap in safety.What Is Partial AUC?
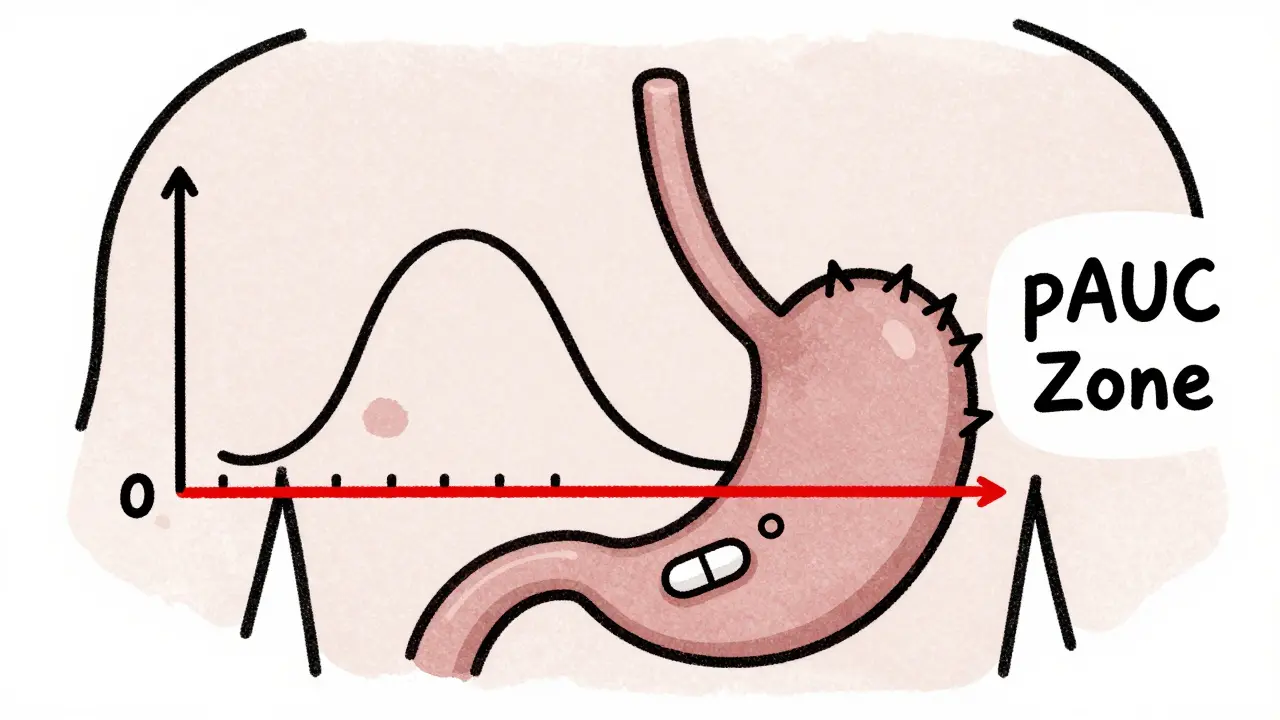
Partial AUC, or pAUC, measures drug exposure only during a specific window of time-usually the early absorption phase where differences matter most. Instead of looking at the whole curve from zero to infinity, you zoom in on the first 1, 2, or 3 hours. Or you pick a window based on when the reference drug hits its peak concentration. Or you focus on the time when concentrations are above 50% of Cmax. The goal? To compare how quickly and consistently the test and reference products deliver the drug when it counts. For a painkiller, that’s the first hour after taking it. For a long-acting blood pressure med, it might be the steady-state window where levels stay above a therapeutic threshold. The U.S. FDA and the European Medicines Agency (EMA) both started pushing for pAUC around 2013. The EMA was the first to formally recommend it for extended-release products. The FDA followed, especially after a 2017 workshop where experts called pAUC an “improved metric” that’s sensitive to differences in high-concentration zones but ignores noise in low-concentration tails.How It Works: The Science Behind the Numbers
Calculating pAUC isn’t just cutting a piece of the curve. There are rules. The FDA says the time window should be tied to a clinically relevant effect. For example, if a drug needs to reach therapeutic levels within 30 minutes to stop a migraine, then the pAUC should cover 0 to 90 minutes. If the reference drug peaks at 4 hours, you might use 0 to 6 hours to capture the full absorption phase. Statistically, you calculate the ratio of test to reference pAUC, then log-transform the data and run an ANOVA-just like you would for regular AUC or Cmax. The result must fall between 80% and 125% to pass bioequivalence. But here’s the catch: pAUC often has more variability. That means you might need more people in your study. A 2020 analysis found that switching to pAUC could require 25-40% more participants than traditional methods. One biostatistician at Teva told the ACPAC forum that for their extended-release opioid generic, they had to increase their study size from 36 to 50 subjects. That added $350,000 to the cost-but it caught a 22% difference in early exposure that Cmax and AUC missed. Without pAUC, that generic might have gone to market and caused overdoses.
Where It’s Used: Real-World Examples
pAUC isn’t for every drug. It’s reserved for complex formulations:- Abuse-deterrent opioids: The FDA requires pAUC to ensure the drug doesn’t release too fast if crushed or snorted. Early exposure must match the brand.
- Extended-release CNS drugs: For Parkinson’s or epilepsy meds, timing is everything. A delay of even 30 minutes can trigger seizures or tremors.
- Mixed-release products: Pills with both immediate and delayed layers. Traditional metrics average out the two phases and hide critical differences.
- Cardiovascular drugs: Especially beta-blockers and antihypertensives where steady levels prevent spikes in heart rate or blood pressure.
Challenges and Criticisms
It’s not perfect. First, there’s no universal rule for picking the time window. One product-specific guidance might say “0 to 2 hours,” another might say “0 to Tmax of the reference.” A 2022 survey found only 42% of FDA guidances clearly defined how to calculate the cutoff. That creates confusion. Generic companies waste months arguing over methodology. Second, it’s expensive. Smaller firms can’t afford the extra subjects or the specialized software (like Phoenix WinNonlin or NONMEM). Many now outsource pAUC analysis to contract research organizations (CROs) like Algorithme Pharma, which now controls 18% of the complex BE study market. Third, regulatory inconsistency. The EMA and FDA sometimes disagree on time intervals. An IQ Consortium report in 2023 found this mismatch adds 12-18 months to global drug approval timelines. That delays affordable medicines. And yes, it’s harder. Biostatisticians need 3-6 months of extra training to get comfortable with pAUC. A 2022 survey found 63% of generic drug teams needed outside statistical help for pAUC-compared to just 22% for traditional metrics.
What’s Next?
The FDA is trying to fix the chaos. In January 2023, they launched a pilot using machine learning to predict the best time window based on reference product data. Instead of guessing, the algorithm learns from thousands of past studies. By 2027, Evaluate Pharma predicts 55% of all new generic approvals will require pAUC. That’s up from 35% in 2022. It’s becoming standard for anything that’s not a simple tablet. The science is solid. As Dr. Bingming Wang, FDA’s Director of Bioequivalence, put it in 2022: “For some products, traditional metrics are not sufficient to ensure therapeutic equivalence.” pAUC isn’t about making things harder. It’s about making sure generics are truly equivalent-not just mathematically, but clinically. Because when a drug doesn’t work the same way, it’s not just a failed study. It’s a patient at risk.What You Need to Know
If you’re a patient: You don’t need to calculate pAUC. But you should know that if your generic drug is for a complex condition-chronic pain, epilepsy, heart disease, or mental health-it’s being held to a higher standard than ever before. If you’re in pharma: pAUC isn’t optional anymore. If your product has modified release, multiple layers, or abuse-deterrent features, you need to plan for it early. Don’t wait until the last minute. Start with pilot studies. Use reference product Tmax. Talk to statisticians. Budget for bigger trials. If you’re a student or researcher: This is where pharmacokinetics is headed. Mastering pAUC means mastering the future of generic drug development.What is partial AUC in bioequivalence?
Partial AUC (pAUC) measures drug exposure during a specific time window-like the first 1-3 hours after dosing-rather than the entire concentration-time curve. It’s used to compare how quickly and consistently a generic drug absorbs compared to the brand-name version, especially for complex formulations like extended-release pills.
Why is pAUC better than total AUC for some drugs?
Total AUC tells you how much drug was absorbed overall, but it ignores when it happened. For drugs that need rapid onset or steady release, timing matters. Two products can have the same total AUC but different absorption rates-one might spike too fast and cause side effects, while another releases too slowly to be effective. pAUC catches those differences.
How is the time window for pAUC chosen?
The time window should be tied to a clinically relevant effect. Common methods include using the reference product’s Tmax (time to peak concentration), focusing on the period where concentrations exceed 50% of Cmax, or selecting a window based on when the drug’s effect begins. The FDA recommends linking it to pharmacodynamic data-like when pain relief starts or when seizure control kicks in.
Do all generic drugs require pAUC?
No. Only complex formulations need it-like extended-release, abuse-deterrent, or mixed-release products. Simple immediate-release tablets still use traditional Cmax and total AUC. As of 2023, the FDA has mandated pAUC for 127 specific drug products, mostly in pain management, CNS disorders, and cardiovascular therapy.
Why does pAUC make bioequivalence studies more expensive?
pAUC often has higher variability, meaning you need more participants to get reliable results. Studies show sample sizes can increase by 25-40% compared to traditional methods. This raises costs for recruitment, blood sampling, and lab analysis. It also requires specialized statistical expertise, which many smaller companies outsource.
Is pAUC used outside the U.S.?
Yes. The European Medicines Agency (EMA) began recommending pAUC in 2013 for prolonged-release formulations. Other regions, including Health Canada and PMDA in Japan, are following suit. But differences in time window rules between regulators can delay global approvals by over a year.
What software is used to calculate pAUC?
The most common tools are Phoenix WinNonlin and NONMEM, both used in pharmacokinetic modeling. These programs allow users to define custom time intervals, apply log-transformation, and calculate confidence intervals using methods like the Bailer-Satterthwaite-Fieller approach. Many CROs now offer pre-built templates for pAUC analysis to streamline submissions.
How do regulatory agencies ensure consistency in pAUC use?
The FDA and EMA publish product-specific guidances that outline when and how to use pAUC. As of 2023, the FDA has over 2,000 such guidances, with about 15% including pAUC. To reduce ambiguity, the FDA launched a pilot in early 2023 using machine learning to recommend optimal time windows based on historical reference product data.