When you take a pill for high blood pressure or antibiotics, chances are it was made in China or India. These two countries produce over 80% of the world’s active pharmaceutical ingredients (APIs) - the core chemical components that make drugs work. But behind that scale lies a critical question: Which country offers safer, more reliable manufacturing? The answer isn’t simple. It’s shaped by regulatory oversight, supply chain vulnerabilities, and decades of policy choices that now determine whether your medicine is safe.
Why FDA Monitoring Matters More Than You Think
The U.S. Food and Drug Administration (FDA) doesn’t just inspect drug plants in America. It sends inspectors to factories in China, India, and dozens of other countries. Why? Because over 40% of all finished pharmaceuticals sold in the U.S. are made overseas, and nearly 80% of APIs come from just two nations: China and India. The FDA’s job isn’t to approve every batch - it’s to ensure the systems in place can consistently produce safe, effective drugs. In 2023, the FDA issued import alerts - which block shipments from entering the U.S. - against 37% of Chinese pharmaceutical facilities. For Indian facilities, that number was 18%. That’s not just a statistic. It means nearly four in ten Chinese plants were flagged for serious quality issues: unclean equipment, falsified data, poor storage conditions, or unapproved process changes. Indian facilities still get flagged, but less often. And when they do, the problems are usually fixable. Chinese facilities, by contrast, often face repeated violations. The difference isn’t luck. It’s structure. India has over 100 FDA-approved manufacturing sites. China has only 28. That’s a 257% gap in certified capacity. Why? Because Indian companies know the FDA’s rules inside and out. They train staff on 21 CFR Part 211 - the FDA’s quality system regulations - from day one. Many have built digital systems that track every step of production, reducing human error. Chinese factories, especially smaller ones, often prioritize speed and cost over documentation. A single missing signature or unverified test result can trigger an FDA alert.India’s Regulatory Edge - And Its Hidden Weakness
India’s strength lies in compliance. Its pharmaceutical industry grew by focusing on generics - cheaper versions of branded drugs - and doing it right. The country’s 1970 Patents Act allowed local production of generics without waiting for patents to expire. That decision, made over 50 years ago, laid the foundation for today’s global dominance. Companies like Dr. Reddy’s, Sun Pharma, and Cipla built their reputations on FDA compliance, not just low prices. Today, over half of all contract research organizations (CROs) in Asia-Pacific are based in India. That’s not an accident. Western drugmakers trust Indian labs to run clinical trials, validate processes, and maintain clean records. The FDA’s inspection reports show Indian plants receive 30% fewer Form 483 observations - the official notices of violations - than Chinese ones between 2020 and 2023. But here’s the contradiction: India is the world’s largest supplier of generic drugs, yet it imports 72% of its APIs from China. That’s up from 66% just two years ago. So while India makes the final pills, it’s relying on China for the raw material. That creates a single point of failure. If a Chinese API plant gets shut down by the FDA, or if trade tensions spike, India’s entire supply chain trembles. One senior procurement manager at a major U.S. drug company told Bain & Company: “We’re trying to diversify, but we can’t make a pill without the API. And right now, China still makes most of it.”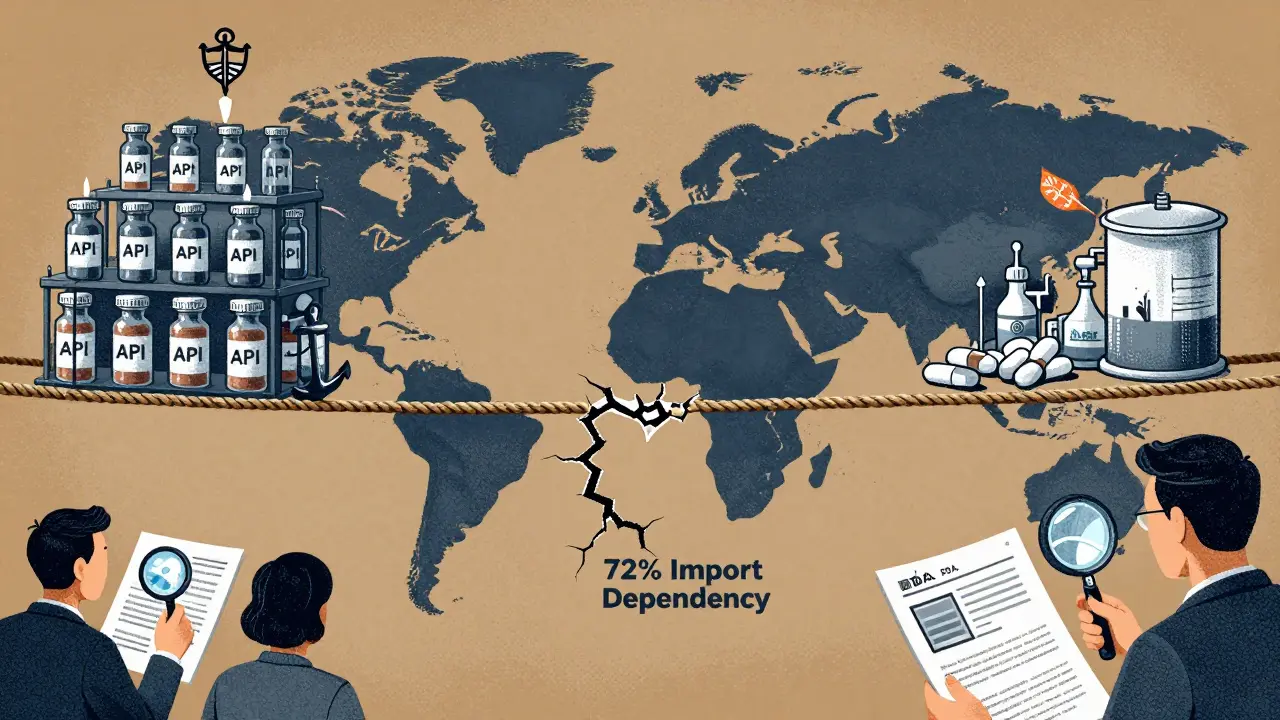
China’s Scale - And Why It’s Becoming Riskier
China dominates volume. It produces about half of all pharmaceutical manufacturing output in Asia. It’s cheaper. Faster. And it’s moving up the value chain - investing billions in biologics, cell therapies, and advanced drug delivery systems. Between 2015 and 2024, China’s biopharmaceutical market grew at a 19.3% annual rate. India’s biosimilars market is growing faster - 22% - but it started from a much smaller base. The problem isn’t capability. It’s consistency. China has thousands of pharmaceutical factories. Only a few hundred are FDA-approved. The rest serve domestic markets or export to countries with looser rules. Many small Chinese manufacturers cut corners to compete on price. A 2025 analysis by AmrepMexico noted that while China has improved its ISO and RoHS certifications, quality control remains uneven across suppliers. Geopolitics is making things worse. The U.S. government is pushing companies to reduce reliance on Chinese manufacturing. Tariffs, export controls, and political pressure have raised the cost of doing business there. Labor costs are rising. The price advantage that once made China irresistible is shrinking. Meanwhile, the FDA has increased inspections and tightened import rules. In 2023, 37% of Chinese facilities faced import alerts. That’s not sustainable for long-term partnerships.The ‘China+1’ Strategy Is Reshaping Global Supply Chains
Global pharmaceutical companies aren’t abandoning China. They’re adding India as a backup. This is called the “China+1” strategy - a deliberate move to avoid putting all your eggs in one basket. The goal isn’t to replace China. It’s to build resilience. India fits perfectly. It speaks English. Its regulatory system is transparent. Its workforce understands FDA requirements. And the government is pouring $3 billion into production-linked incentives (PLIs) to boost domestic API manufacturing. The revised Schedule M regulations, updated in 2023, are pushing Indian factories toward global standards - especially for specialty generics and complex injectables. But India’s manufacturing base is fragmented. You don’t work with one giant plant. You work with dozens of smaller suppliers. That means more coordination, more audits, more paperwork. In China, you might deal with one integrated hub. In India, you need a team just to manage your vendor list. Setting up in India takes longer - 6 to 9 months to align with FDA standards - compared to 3 to 6 months in China. But here’s the catch: Chinese facilities often need costly remediation after the initial approval. Indian plants, once compliant, tend to stay compliant.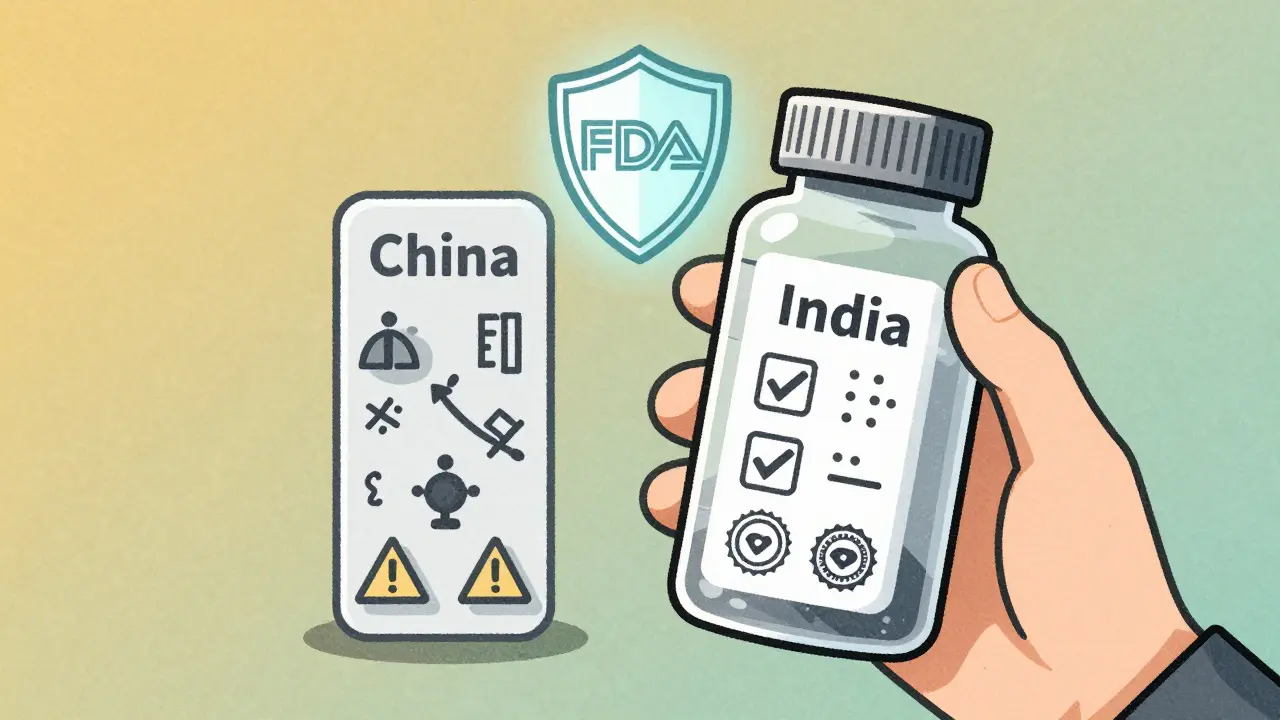
Who Wins - and Who Pays the Price?
For patients, the winner is clarity. If your medicine comes from an FDA-approved Indian plant, it’s more likely to meet global quality standards. If it’s made in a Chinese facility without FDA oversight, you’re relying on a different set of rules. For drugmakers, India is becoming the default choice for high-risk, high-value products: antibiotics, heart medications, cancer drugs. China still dominates low-cost, high-volume generics - especially for markets in Africa, Latin America, or Southeast Asia where regulatory scrutiny is lighter. But the real cost isn’t just financial. It’s risk. A contaminated batch of metformin in 2020 traced back to an unapproved Chinese API supplier led to recalls across five continents. A similar incident in India would be far less likely - not because Indian companies are perfect, but because their systems are built to catch errors before they leave the plant. The future belongs to companies that can balance cost, scale, and compliance. India’s advantage isn’t just in its factories. It’s in its culture of accountability. China’s advantage is still in its capacity. But as FDA scrutiny grows and geopolitical risks rise, that capacity is becoming a liability.What This Means for You
You don’t need to know which factory made your pill. But you should know this: the system that checks it matters. The FDA doesn’t test every batch. It tests the system. And right now, that system works better in India than in China. If you’re a patient, your best protection is choosing brands that disclose their manufacturing partners. If you’re a health professional, ask where the APIs come from. If you’re a policymaker or investor, the data is clear: India’s compliance edge is real - but only if it reduces its dependence on Chinese inputs. The next decade will decide whether the world’s medicines are made by companies that prioritize safety - or just speed. Right now, India is betting on safety. China is betting on scale. One of them will lead the next generation of global pharma. The other might be left behind.Why does the FDA inspect factories in India and China?
The FDA inspects foreign factories because over 40% of finished drugs and 80% of active ingredients used in U.S. medicines are made overseas. The FDA doesn’t just approve drugs - it ensures the manufacturing systems meet U.S. quality standards, no matter where they’re located. Without these inspections, there’s no way to guarantee safety.
Is Indian-made medicine safer than Chinese-made medicine?
On average, yes. Indian manufacturing facilities receive fewer FDA violations and are more likely to be FDA-approved. Over 100 Indian plants have FDA clearance, compared to just 28 in China. Indian companies are also more likely to use digital systems to track quality, reducing human error. However, India relies on China for 72% of its raw ingredients, which creates a hidden risk.
What is the China+1 strategy in pharmaceuticals?
The China+1 strategy means sourcing critical products from China, but also building backup supply chains - usually in India - to avoid disruption. After years of relying mostly on China, U.S. and European drugmakers now see the risk. If a Chinese factory gets shut down by the FDA or affected by trade tensions, having a compliant alternative in India keeps medicines flowing.
Why does India import so many APIs from China?
India lacks the scale and infrastructure to produce all its own active ingredients at competitive prices. China has invested heavily in bulk chemical production for decades and can make APIs cheaper and faster. India’s focus has been on formulation - turning those ingredients into pills and injections. But that dependency is now seen as a major supply chain vulnerability.
Are FDA-approved drugs from India always safe?
No approval is a guarantee of perfection. Even FDA-approved plants can have occasional lapses. But FDA approval means the facility has passed rigorous inspections and has systems in place to catch and correct errors. The odds of a serious quality issue are far lower than with non-approved facilities. FDA approval is a signal of reliability - not perfection.






13 comments
Nikki Brown
Wow. Just... wow. I can't believe we're still letting China poison our medicine with their shoddy labs and falsified data. 😤 Our FDA is doing God's work, but it's not enough. We need a full embargo. No more APIs from Beijing. Not one pill. Not one capsule. Our children's lives are not a bargaining chip for cheap generics. 🇺🇸💊
Peter sullen
It is imperative to acknowledge, with rigorous analytical precision, that the regulatory architecture underpinning pharmaceutical manufacturing in India demonstrates a statistically significant superiority in terms of compliance with 21 CFR Part 211, vis-à-vis the PRC’s heterogeneous, fragmented, and often non-conforming production ecosystems. The FDA’s 37% import alert rate against Chinese facilities-compared to 18% for India-is not merely indicative; it is a structural alarm bell.
Furthermore, the institutionalized adoption of digital traceability systems, validated change control protocols, and GMP-certified workforce training in Indian facilities creates a multi-layered quality assurance framework that is, frankly, absent in the majority of Chinese subcontractors. This is not a matter of ‘luck’-it is a matter of systemic design.
Amy Lesleighter (Wales)
Look, I’m not a scientist, but I know this: if your pill’s made in a place that can’t even keep its lab clean, you shouldn’t be taking it. India’s got its flaws, sure-but at least they’re trying to fix them. China? They’re still cutting corners to save a buck. And yeah, we rely on them for the stuff inside the pill... but that’s like buying a car from a guy who only makes the screws. You don’t trust him with the whole thing.
Becky Baker
India? Yeah, sure. But let’s not pretend they’re saints. They’re just better at pretending. Meanwhile, China’s building the future-biologics, mRNA, AI-driven drug design. We’re clinging to FDA checklists like they’re holy scripture while the world moves on. If you’re scared of Chinese medicine, maybe you’re scared of progress.
Rajni Jain
As someone from India, I want to say thank you for recognizing our work. We’ve worked so hard to earn FDA trust. But you’re right-we’re too dependent on China for APIs. It hurts. We make the pills, but we can’t even make the medicine inside. We’re trying to change that. The PLI scheme? It’s real. We’re not giving up. Just... give us time.
Natasha Sandra
China’s getting a bad rap, but let’s be real-India’s not perfect either. 😕 I’ve seen the reports. But I’ll take an Indian-made pill any day over a Chinese one with a 1-in-3 chance of being flagged. 🤞💊 Still... why are we letting our meds be a geopolitical pawn? Someone’s gotta fix this.
Erwin Asilom
There’s a deeper issue here: we’ve outsourced the foundation of our healthcare to two nations with wildly different governance models. The FDA can inspect, but it can’t control. The real solution isn’t choosing between China and India-it’s rebuilding domestic API capacity. It’s expensive. It’s slow. But it’s the only path to true sovereignty.
Sumler Luu
I appreciate the nuance in this post. It’s easy to villainize China, but the truth is more complicated. India’s compliance edge is real, but it’s built on a fragile supply chain. Maybe the answer isn’t ‘India over China’-but ‘neither, alone.’ We need more global partners. Vietnam. Mexico. Even the EU. Diversify or die.
sakshi nagpal
As an Indian professional in pharma, I see both sides. We’re proud of our compliance culture, but we’re also painfully aware of our dependency. China’s scale is undeniable. Their R&D investment in biologics is staggering. Maybe we should stop seeing this as a rivalry and start seeing it as a partnership-with better oversight. We need each other. But we also need transparency.
Sandeep Jain
bro... we all know china makes the stuff but india makes it into pills. but like... who checks the stuff before it gets to india? no one really. its a mess. and yeah, we got a lot of factories but most are tiny. one bad batch and the whole chain shakes. not cool.
roger dalomba
Wow. A 2,000-word essay on pill manufacturing. And yet, the only thing that matters is: does it work? If your blood pressure’s under control, shut up and take your medicine.
Brittany Fuhs
India? Please. They’re just better at lying to inspectors. China’s got the infrastructure. The workforce. The ambition. We’re clinging to a 1950s regulatory fantasy while the rest of the world builds the future. And don’t even get me started on how we’re outsourcing our health to a country that doesn’t even let its own people breathe clean air.
Sophia Daniels
Let’s be real: China’s playing 4D chess. They know we’re addicted to their APIs. So they’re slowly weaponizing it. Every FDA alert? A strategic distraction. Every tariff? A chance to raise prices. And India? They’re the middleman in a game they didn’t design. We’re not choosing between two countries-we’re choosing between two forms of exploitation. And guess what? The patient always pays.