Tablet Capping: Why Pills Crack and How Manufacturing Fixes It
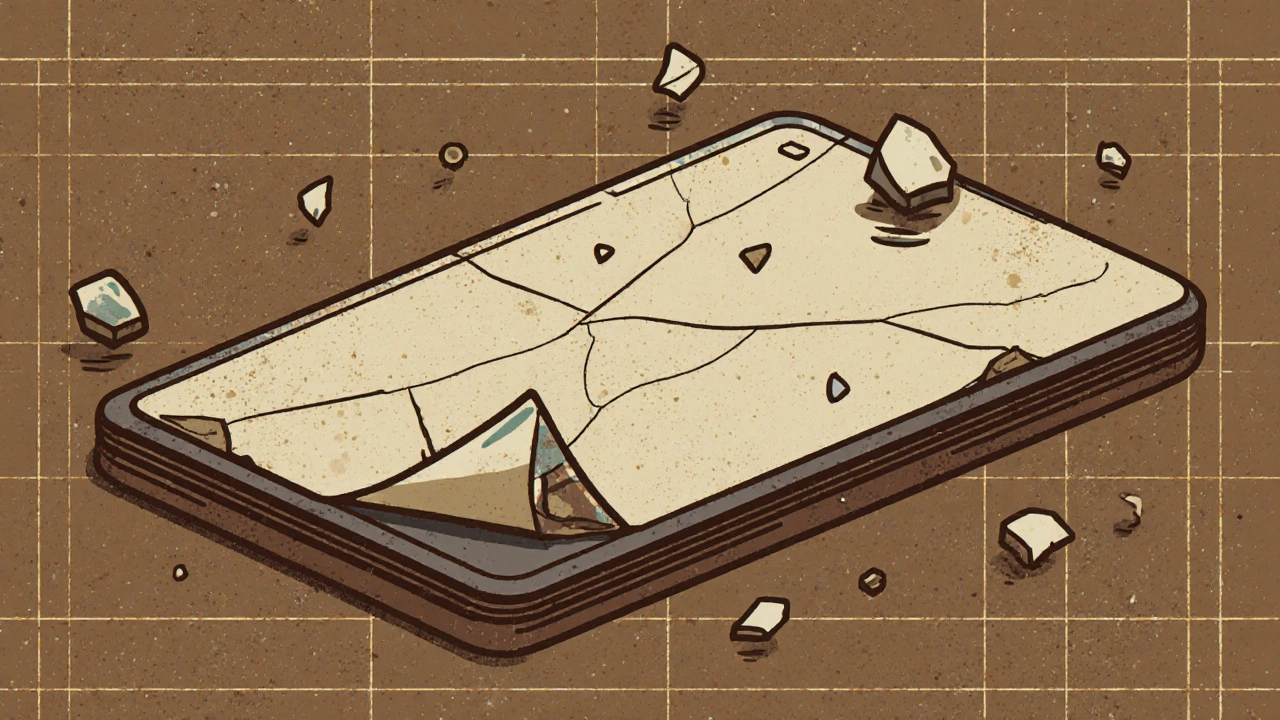
When a tablet splits cleanly along the top edge—like a cookie broken in half—that’s tablet capping, a manufacturing defect where the upper layer of a compressed pill separates from the body. It’s not just ugly—it means the dose could be uneven, the drug might not dissolve right, or worse, the tablet could break apart in your hand or pill organizer. This isn’t rare. In fact, it’s one of the top three tablet defects reported in pharmaceutical plants worldwide, and it happens at every scale—from small labs to big factories making billions of pills a year.
Tablet manufacturing, the process of compressing powder into solid pills is more delicate than it looks. The powder blend has to flow just right, the granules need the perfect moisture level, and the punch pressure must be timed exactly. Too much pressure? The tablet gets too hard and cracks under stress. Too little? It falls apart before it even leaves the machine. pharmaceutical quality, the system of checks that ensures every pill works as intended exists because of problems like capping. If a batch fails, it’s not just tossed—it’s traced back to the granulator, the blender, even the humidity in the room.
Why should you care? Because a capping issue doesn’t always show up on the label. You might take a pill that looks fine, but if the top layer separated during packaging or shipping, you could be getting less medicine than you think. That’s why manufacturers run constant tests: hardness testers, friability machines, and visual inspections under bright lights. Even small changes—like switching a binder ingredient or moving the production line to a new building—can trigger capping.
It’s not just about the machine. The powder itself matters. If the particles are too fine, they don’t stick well. Too coarse, and they don’t compress evenly. Moisture is another silent killer—too much, and the powder sticks to the punch; too little, and it crumbles. That’s why skilled technicians don’t just watch the machines—they smell the powder, feel the texture, and track every batch like a detective.
What you’ll find in the posts below isn’t a list of fixes—it’s a look at how real-world drug quality works. From calibration tools that keep presses accurate, to how global factories handle the same problem under different rules, to why some generic pills are more likely to cap than others. These aren’t theory papers. They’re stories from the floor, the lab, and the inspection line—where tablets are made, tested, and sometimes rejected before they ever reach your medicine cabinet.
- Nov 26, 2025
- Posted by Cillian Osterfield
Common Manufacturing Defects in Generic Drugs and How They Impact Safety
Generic drugs save money but often have higher manufacturing defect rates than brand-name versions. Common issues like capping, contamination, and weight variation can affect safety and effectiveness. Learn what causes these defects and how to spot them.
Categories
- Health and Wellness (72)
- Medications (69)
- Health and Medicine (28)
- Pharmacy Services (12)
- Mental Health (9)
- Health and Career (2)
- Medical Research (2)
- Business and Finance (2)
- Health Information (2)
©2026 heydoctor.su. All rights reserved