When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But behind that simple tablet lies a complex manufacturing process where small errors can have serious consequences. Between 2019 and 2023, generic drugs had 3.2 times more quality defects than their branded counterparts, according to FDA inspection data. These aren’t just cosmetic flaws-they’re safety risks that can lead to underdosing, overdosing, or even life-threatening reactions.
What Are the Most Common Manufacturing Defects?
Generic drug manufacturers face pressure to cut costs, and that often means using older equipment, skipping advanced quality controls, or running production lines faster than they were designed for. The result? Specific, well-documented defects that show up again and again.- Capping: The top or bottom of a tablet splits off horizontally. This happens when compression force exceeds 15 kN and the tablet’s moisture content drops below 2%. It’s common in hydrophobic drugs like certain pain relievers and antidepressants.
- Lamination: Layers peel apart inside the tablet. This occurs when turret speeds go above 40 rotations per minute and pre-compression is too weak. It’s especially risky in extended-release formulations where the drug must release slowly over hours.
- Sticking: The active ingredient sticks to the machine’s punch heads. This happens when APIs with melting points below 120°C are compressed in humid conditions. Moisture levels above 4% during long runs can increase ejection forces by 300-500 N, causing tablets to deform or break.
- Mottling: Uneven color patterns on the tablet surface. While mostly cosmetic, severe mottling signals inconsistent mixing of ingredients, which can mean inconsistent dosing.
- Weight variation: Tablets fall outside the allowed weight range. The USP <905> standard permits no more than 5% deviation. When granule flow rates drop below 0.5 g/s, 12.7% of batches fail this test-leading to dangerous under- or overdosing.
- Particulate contamination: Tiny particles of metal, glass, or foreign material end up in injectables. This is the leading cause of recalls for generic IV drugs and can trigger immune reactions or block blood vessels.
These defects aren’t random. They’re the direct result of outdated machinery, poor environmental controls, and insufficient staff training. A 2023 FDA report found that 24% of all drug recalls were tied to violations of Current Good Manufacturing Practices (CGMP)-and nearly two-thirds of those came from generic manufacturers.
Why Are Generic Drugs More Prone to Defects?
It’s not that generic manufacturers are careless. It’s that the business model makes quality investment difficult.Branded drug companies spend 15-18% of their production costs on quality assurance. Generic manufacturers? Only 8-10%. Why? Because they compete on price. A single generic pill might sell for pennies. To stay profitable, companies cut corners-delaying equipment upgrades, reducing testing frequency, or sharing production lines between multiple drugs.
Shared facilities are a major problem. When one product is manufactured on a line, residue can linger. If the next product is a hormone or a potent steroid, even microscopic contamination can cause serious side effects. In 2022, a batch of generic testosterone gel was recalled after traces of a blood pressure medication were found in it.
And the infrastructure is aging. Many generic plants in the U.S. and India were built in the 1980s or 1990s. They lack modern sensors, automated inspection systems, and real-time monitoring. One study showed that human visual inspection misses 30% of defects. Automated systems can catch 98%-but they cost hundreds of thousands of dollars. Most small manufacturers can’t afford them.
Who Gets Hurt?
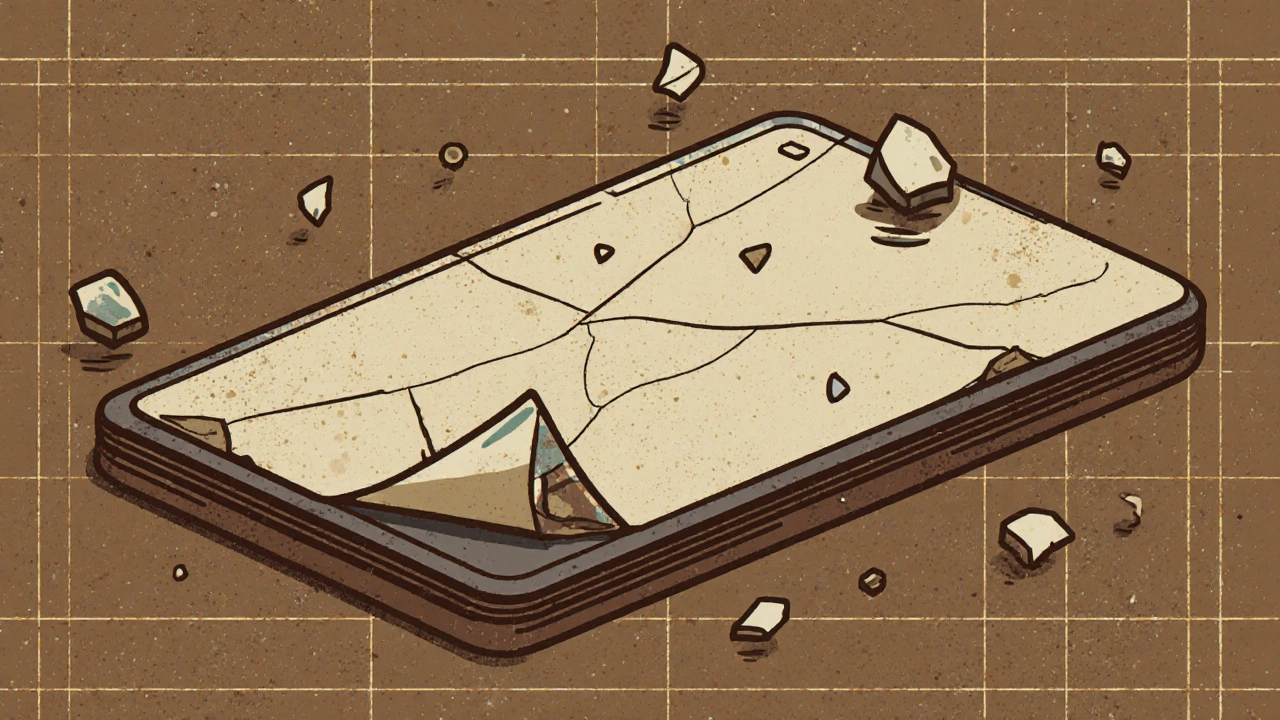
Patients don’t always know when a generic drug is defective. A cracked tablet might look odd, but what if the dose inside is off by 20%? That’s enough to make a blood thinner ineffective-or a thyroid medication cause heart palpitations.Pharmacists report the problem is getting worse. A 2023 survey of 1,247 U.S. pharmacists found that 68% had encountered quality issues with generics in the past year. Nearly half had patients complain about tablets crumbling, changing color, or tasting different. One pharmacist in Ohio described receiving a batch of metformin ER tablets that turned to dust when handled. Patients who took them reported spikes in blood sugar.
The FDA’s MedWatch system recorded 1,842 adverse events in 2023 directly linked to generic drug quality. Over 300 involved visible defects-chipped, cracked, or discolored pills. But many more go unreported. Patients assume their medication is working normally, even if they feel different. A 2021 study in JAMA Internal Medicine found that 7.3% of generic drug applications failed bioequivalence tests-not because the formula was wrong, but because manufacturing inconsistencies changed how the drug was absorbed.

What’s Being Done to Fix It?
Regulators are pushing for change. The FDA’s 2022 Quality by Design (QbD) guidelines require manufacturers to map out the exact conditions under which a drug can be safely made-called the “design space.” If a company wants to change a process, they must prove it still meets safety standards.Some manufacturers are stepping up. The FDA’s Emerging Technology Program has helped 47 generic companies switch to continuous manufacturing. Unlike old batch methods, continuous systems run 24/7 with real-time monitoring. Defect rates drop by 65%.
AI-powered visual inspection systems are also making a difference. At companies like Sandoz and Dr. Reddy’s, these systems scan every tablet at 600 per minute, spotting defects as small as 0.1 mm. They catch 92% of problems-far better than human inspectors.
But progress is slow. The Generic Pharmaceutical Association estimates it would take $28.7 billion to upgrade all U.S. generic manufacturing facilities to modern standards. Right now, the industry invests only $1.2 billion a year. That gap is widening.
What Should You Do as a Patient?
You can’t control the factory, but you can stay alert.- Check your pills. If they look different-different color, shape, size, or texture-ask your pharmacist. Don’t assume it’s just a different batch.
- Keep track of how you feel. If a generic drug suddenly seems less effective or causes new side effects, talk to your doctor. It could be a quality issue.
- Report problems. Use the FDA’s MedWatch system to report defective pills. Your report helps regulators spot patterns before more people are affected.
- Ask about alternatives. If you’ve had issues with one generic brand, your doctor may be able to prescribe a different manufacturer’s version. Not all generics are made the same.
Some patients with chronic conditions-like epilepsy, thyroid disease, or heart failure-are especially vulnerable. For them, even a tiny change in drug absorption can be dangerous. If your doctor recommends switching generics, ask: “Is this the same manufacturer as before?” If they don’t know, it’s worth finding out.
The Bigger Picture
Generic drugs make up 90% of prescriptions in the U.S. but only 23% of spending. That’s why they exist-to save money. But when quality slips, the cost isn’t just financial. It’s measured in hospital visits, lost productivity, and lives.Regulators know this. The FDA’s 2024-2027 plan aims to cut quality-related drug shortages by 30%. But real change needs investment, not just rules. Without better equipment, better training, and better oversight, the same defects will keep showing up.
For now, the system works-most of the time. But when it fails, the consequences are real. And they’re avoidable.
Can generic drugs be less effective than brand-name drugs?
Legally, generics must be bioequivalent-meaning they deliver the same amount of active ingredient into the bloodstream at the same rate. But manufacturing inconsistencies can cause real-world differences. A 2021 study found that 7.3% of generic applications failed bioequivalence tests not because of the formula, but because of how the drug was made. If a tablet crumbles, has uneven coating, or inconsistent granule size, absorption can vary-leading to underdosing or overdosing.
Are generic drugs made in the same facilities as brand-name drugs?
Sometimes, but not always. Many brand-name companies outsource production to third-party manufacturers, and those same facilities often make generics too. The problem is that when a line switches from one drug to another, residue can remain. That’s why shared facilities are a major source of contamination. Even if the same company makes both versions, the generic version may be produced on older equipment or with less rigorous controls.
How can I tell if my generic drug has a quality defect?
Look for changes in appearance: different color, shape, size, or texture. Tablets that crumble, chip, or have visible cracks are red flags. If the pill tastes different or doesn’t dissolve as expected, that’s also worth noting. If you’re unsure, ask your pharmacist to check the batch number and compare it to previous fills. You can also report suspicious pills to the FDA’s MedWatch system.
Why do some generic drugs cause different side effects?
Inactive ingredients-like fillers, dyes, or coatings-can vary between manufacturers. These don’t affect the drug’s action, but they can change how fast it’s absorbed or trigger reactions in sensitive people. For example, a dye in one generic version might cause headaches in someone allergic to it. Also, if the active ingredient isn’t evenly distributed due to poor mixing, some pills may contain too much or too little-leading to unexpected effects.
Is it safe to switch between different generic brands?
For most people, yes. But for drugs with narrow therapeutic windows-like warfarin, levothyroxine, or seizure medications-even small changes in absorption can be risky. If you’ve been stable on one generic brand, switching to another without medical supervision could cause problems. Always talk to your doctor before switching, especially if you’re on a critical medication.
What’s Next for Generic Drug Safety?
The future depends on technology and regulation. Continuous manufacturing, AI inspections, and blockchain-based tracking are becoming more common. The 2024 Drug Supply Chain Security Act is helping reduce counterfeits. But without more funding, progress will be slow.Right now, the system is a balancing act: keeping drugs affordable while ensuring they’re safe. Too much regulation drives up prices. Too little risks lives. The challenge isn’t just about fixing machines-it’s about changing a system that rewards low cost over high quality. Until that changes, patients will keep seeing the same cracked tablets, the same inconsistent effects, and the same quiet warnings from pharmacists who’ve seen it all before.





12 comments
Jauregui Goudy
Okay, let’s be real-this isn’t just about pills falling apart. It’s about a system that treats human lives like a spreadsheet line item. I’ve seen pharmacists shake their heads over batches of metformin that crumble like chalk. And no one’s talking about how the FDA’s inspection schedule is laughably outdated. They show up once every 3-5 years? Meanwhile, plants run 24/7 with half-trained staff and machines older than some interns. This is systemic neglect dressed up as cost-saving.
And don’t even get me started on the ‘bioequivalent’ lie. Sure, the math says it’s the same. But if your tablet dissolves in 20 minutes instead of 80 because the coating’s thin as tissue paper? Your blood levels spike and crash. That’s not equivalence-that’s roulette with your health.
shawn monroe
As a former QA engineer at a generics plant in Indiana, I can tell you: the numbers are underreported. We had a line that produced 12M tablets/month. Visual inspection missed 28% of capping defects. Automated systems? We begged for one. Management said ‘We’re not Apple, we’re a dime-a-pill operation.’ We had one guy with a magnifying glass and a clipboard checking 500 tablets/hour. He got promoted to ‘senior inspector’ after he passed out from eye strain.
The real kicker? The same plant made both brand-name and generic versions. The brand got new equipment. The generic got the 1998 press with a broken pressure sensor. Guess which one got recalled? The brand version had zero defects. The generic? 3 recalls in 18 months. And no one blinked.
TL;DR: It’s not the formula. It’s the factory. And the factory doesn’t care until someone dies.
Asha Jijen
this is so crazy why dont they just make better pills like the fancy ones i mean its just medicine right
Sue Haskett
Thank you for writing this with such clarity. I’m a nurse, and I’ve had patients come in with tablets that literally dissolved in their palms. One woman with epilepsy had a seizure after switching to a new generic-her blood levels dropped 40%. She didn’t know why. No one told her to check the pill’s appearance. We need public education, not just regulatory patches. This isn’t just a manufacturing issue-it’s a public health literacy crisis.
Also, please don’t assume all generics are equal. I’ve seen the same drug from three different manufacturers, and two were fine. One looked like it was pressed from crushed cereal. The pharmacist didn’t even notice. That’s the problem: we’ve normalized it.
Patients need to know: if it looks weird, it probably is. Don’t take it. Don’t just swallow it because it’s cheaper. Your body isn’t a budget line.
marie HUREL
I’ve been on levothyroxine for 12 years. I’ve switched generics five times. Three times, I felt off-fatigue, heart palpitations, brain fog. I thought it was stress. Then I read about bioequivalence variability. I started keeping a journal: pill color, shape, date, how I felt. Turns out, the blue oval one from Company X made me feel fine. The white round one from Company Y? I was a wreck for three weeks. I switched back. My doctor didn’t even know the difference between the brands. He just said, ‘It’s the same thing.’
It’s not. And no one’s telling patients this. We’re being treated like lab rats in a pricing experiment.
Emma Dovener
As someone who works in global supply chains for pharmaceuticals, I can confirm: the majority of generic manufacturing is outsourced to countries with lower labor and regulatory costs. The FDA inspects less than 10% of foreign facilities annually. Many plants operate with minimal documentation. One facility I visited had handwritten batch logs. No digital traceability. No real-time monitoring. Just a guy with a clipboard and a prayer.
And yet, we trust these pills to treat cancer, heart disease, epilepsy. The disconnect is staggering. We demand cheap drugs, then act shocked when they fail. We’re complicit.
The solution isn’t just more inspections. It’s transparency. Every pill should have a QR code linking to its manufacturing history-date, location, equipment used, QA results. If that were mandatory, companies would invest in quality. Because consumers would know.
Lauren Zableckis
My mom takes blood thinners. She once got a batch that was slightly darker. She didn’t say anything. Two weeks later, she bruised from a bump on her arm that should’ve been a tiny welt. We found out later the batch had inconsistent warfarin distribution. She’s fine now, but I think about how many people never connect the dots. This isn’t paranoia. It’s pattern recognition. If your medication feels ‘off,’ it probably is. Trust yourself.
Jonah Thunderbolt
Oh wow. Another ‘generic drugs are evil’ hot take. Let me guess-you also think vaccines are a plot by Big Pharma and gluten is a government conspiracy? 🙄
Look, I get it. You want to feel like a medical detective. But 99% of generics are perfectly safe. The FDA doesn’t sleep. And if you’re so concerned, pay the extra $10 for the brand. But don’t scare people with cherry-picked horror stories. Most of these ‘defects’ are cosmetic. People are dying from opioid overdoses, not from cracked tablets.
Also, why do you think generics exist? To save lives by making treatment affordable. You want perfection? Then pay $500 for a month’s supply. But don’t blame the system for giving the poor a chance to live. 🤦♂️
Rebecca Price
Jonah, your comment is the textbook definition of privilege in action. You say ‘pay the extra $10’-but what if you’re on Medicaid? What if you’re choosing between insulin and groceries? What if you’re a single parent working two jobs and your pharmacy swaps your pill without telling you? You think this is about choice? It’s not. It’s about survival.
And yes, these ‘cosmetic’ defects? They’re not cosmetic. A tablet that crumbles doesn’t just look bad-it delivers 20% less drug. That’s not ‘cherry-picked.’ That’s documented in FDA reports. You’re not a medical expert. You’re a comment section philosopher who thinks empathy is a weakness.
Maybe if you’d ever had to explain to your child why their seizure meds suddenly stopped working, you’d understand why this isn’t just ‘hot takes’-it’s life-or-death.
Tom Shepherd
i had a generic adderall once that tasted like chalk and made me dizzy for a week. i thought i was just weird but now i get it. also the batch number changed but no one told me. dumb.
Aishwarya Sivaraj
It’s strange, isn’t it? We trust our phones to track our steps, our cars to park themselves, but we hand over our lives to a tablet made by a machine that hasn’t been upgraded since the Clinton administration. We demand innovation everywhere except where it matters most: in the medicine that keeps us alive.
Maybe the real defect isn’t in the pill-but in our collective silence. We accept it because we’re told to. Because ‘it’s cheaper.’ But cheapness isn’t a virtue when it’s paid for in health.
I think we need to stop asking ‘Is this safe?’ and start asking ‘Why is this allowed?’
Rebecca Price
And to everyone else: thank you for speaking up. This isn’t about being anti-generic. It’s about being pro-safety. We can have affordable medicine without sacrificing dignity. But only if we refuse to look away.