Every year, more than 90% of prescriptions filled in the U.S. are for generic drugs. They’re cheaper, widely available, and trusted. But behind that reliability is a supply chain hanging by a thread. As of April 2025, there were 270 active drug shortages in the U.S.-and nearly all of them involved generic medications. These aren’t rare glitches. They’re systemic failures rooted in how these drugs are made, priced, and moved across the globe.
Why Generic Drugs Are the Weakest Link
Generic drugs make up just 13.1% of total U.S. drug spending, yet they’re used in the vast majority of prescriptions. That’s because they’re cheap. But low price means thin profit margins. A single vial of a generic IV antibiotic might cost $2. A chemotherapy drug like cisplatin? Maybe $5. For manufacturers, that’s barely enough to cover labor, packaging, and compliance. When something goes wrong-a power outage, a quality inspection failure, a natural disaster-there’s little room to absorb the cost.Compare that to brand-name drugs. Those companies have higher margins. They invest in multiple suppliers, stockpile inventory, and spread production across continents. Generic manufacturers? They often rely on one factory in India or one API plant in China. If that one source shuts down, the entire U.S. supply can vanish.
The Global Bottleneck: Where APIs Come From
The heart of every drug is the active pharmaceutical ingredient, or API. For generic drugs, about 40% of global API production comes from China. India handles most of the finished dosage forms-tablets, injections, suspensions. Together, these two countries supply the bulk of the world’s low-cost generics.But this concentration creates massive risk. The FDA has documented a history of unreliable manufacturing practices in some overseas facilities. In 2023, a tornado destroyed a Pfizer plant in Kentucky, knocking out 15 essential medications. In 2024, quality violations in an Indian facility shut down cisplatin production nationwide. No one was to blame for the tornado. But the fact that only a handful of companies could make cisplatin? That was by design.
Even more troubling: many of these foreign manufacturers don’t submit complete Drug Master Files (DMFs) to the FDA. Why? Because they’re hesitant to share proprietary processes with a regulator that’s underfunded and stretched thin. The result? A system where the U.S. depends on foreign factories it can’t fully inspect or control.
Sterile Injectables: The Most Vulnerable
Not all generic drugs are equally at risk. Sterile injectables-IV fluids, antibiotics, anesthetics, chemotherapy drugs-are the most likely to go missing. Why? Because they’re hard to make.Producing a sterile injection requires cleanrooms, aseptic processing, specialized equipment, and months of validation. A single contaminant can ruin an entire batch. These drugs also have short shelf lives and can’t be easily stockpiled. And they’re often made by just one or two manufacturers. When one fails, there’s no backup.
In 2024, shortages of epinephrine, heparin, and saline solutions forced hospitals to ration care. Patients had to wait for surgery. Cancer treatments were delayed. Emergency rooms used expired IV bags because newer ones weren’t available. These aren’t hypotheticals. They’re happening right now.
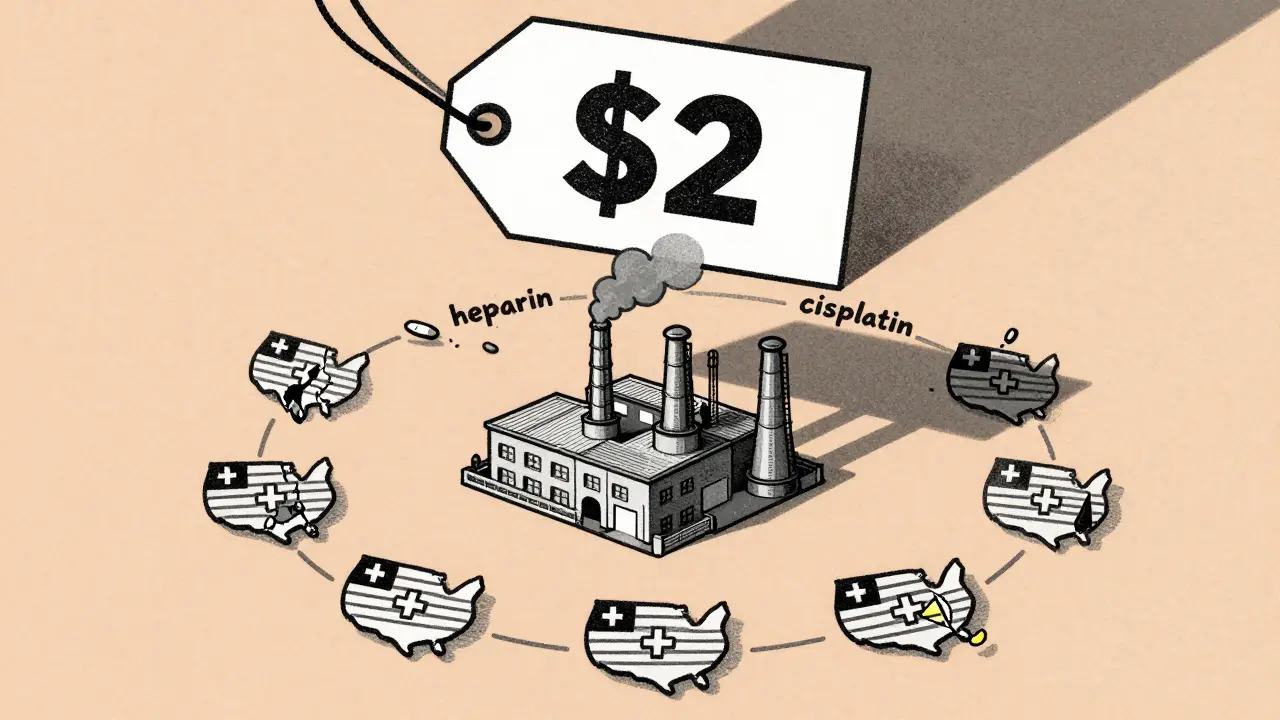
The Economic Trap: Low Prices, High Risk
The biggest driver of shortages isn’t war, pandemics, or politics. It’s price.Generic drug prices have been dropping for decades. Retailers, insurers, and government programs like Medicaid push for the lowest possible bid. Manufacturers compete on cost, not quality or reliability. The winner? The company that can produce the drug cheapest-even if it means cutting corners on maintenance, staffing, or quality control.
Dr. Malta of the U.S. Pharmacopeia (USP) put it plainly: “There’s a clear correlation between drug price and shortage risk.” Older generics, especially those made decades ago, are the most vulnerable. Why? Because no one’s willing to pay more for them. So manufacturers leave the market. Production consolidates. Then, when the last remaining factory has a problem? No one else can step in.
One hospital pharmacist told Pharmacy Times they spend 20-30% of their workweek managing shortages. That’s not filling prescriptions. That’s calling suppliers, tracking down alternatives, compounding drugs by hand, and explaining to patients why their medication isn’t available.
Why Onshoring Won’t Fix This Alone
Some lawmakers argue the answer is to bring drug manufacturing back to the U.S. But it’s not that simple.Rebuilding domestic API and sterile injectable capacity would take 5-7 years and cost $20-30 billion. There aren’t enough trained workers. Regulatory approval for new facilities takes years. And even if you build them, who will operate them profitably? A U.S.-made generic antibiotic might cost $15 instead of $2. Insurers won’t pay that. Hospitals won’t stock it.
CSIS analysts warn that imposing tariffs-some proposals suggest 50-200%-on imported APIs could make things worse. Higher costs mean fewer manufacturers can compete. Fewer competitors mean more consolidation. More consolidation means more single points of failure.
There’s no magic bullet. You can’t just “make it in America” and call it solved.
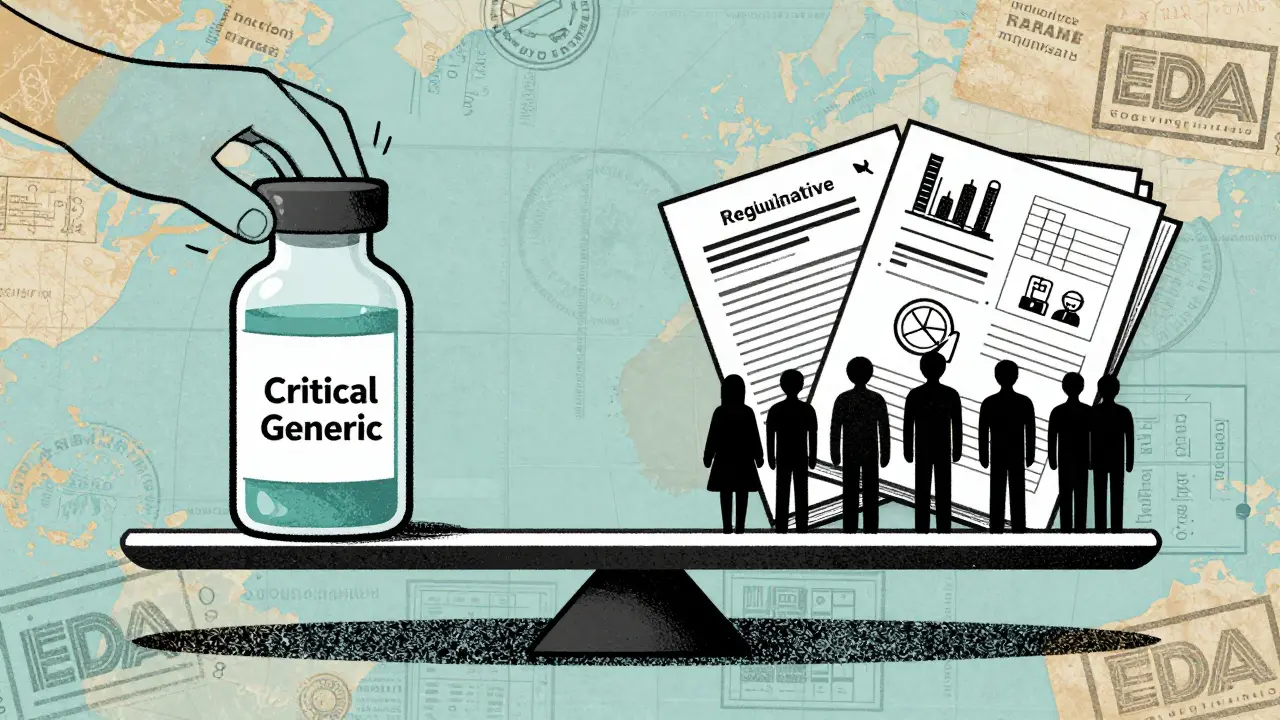
What’s Being Done-and Why It’s Not Enough
There are proposals. S.2062, introduced in 2025, would require manufacturers to maintain six-month reserves of critical generic drugs. The American Hospital Association supports it. So do pharmacists. But it’s stalled in Congress.The FDA has increased inspections of foreign facilities. But domestic inspection capacity has shrunk due to budget cuts and staff reductions across HHS. That means more oversight overseas, less at home. The result? A regulatory blind spot.
Some states are trying to create their own stockpiles. Others are pushing for transparent labeling that shows where APIs come from. But without federal coordination, these efforts are patchwork. A shortage in Ohio doesn’t stop a shortage in California.
The Association of Accessible Medicines continues to push for policies that ensure sustainable pricing. But without real financial incentives-like guaranteed minimum prices for essential generics-manufacturers have no reason to invest.
What This Means for Patients
This isn’t a supply chain issue. It’s a patient safety issue.Imagine needing antibiotics for an infection. Your doctor prescribes a generic. But the pharmacy doesn’t have it. You wait three days. The infection spreads. You end up in the ER.
Or you’re undergoing chemotherapy. Your treatment is delayed because the IV drug isn’t available. The cancer progresses. The window for effective treatment closes.
These aren’t rare stories. The USP 2025 Annual Drug Shortage Report documents dozens of cases where patients suffered harm because their medication wasn’t there. Internal medicine, oncology, pediatrics, emergency care-all hit hard.
And the worst part? It’s predictable. We’ve seen this pattern before. We know which drugs are at risk. We know which manufacturers are the only ones making them. We know the prices that make it unsustainable.
Yet we keep doing the same thing: waiting for the next shortage to happen, then scrambling to fix it.
The Way Forward
Fixing this requires three things: financial stability, diversification, and transparency.- Financial stability: The government needs to set minimum prices for critical generic drugs-especially sterile injectables. Not high prices. Just enough to cover cost, quality, and a small profit. That gives manufacturers a reason to stay in the game.
- Diversification: Encourage more manufacturers to enter the market. Offer tax credits, streamlined approvals, and shared infrastructure for sterile production. Don’t rely on one factory in India or China. Spread it out.
- Transparency: Require labeling that shows the origin of APIs. Make this data public. If a drug’s API comes from a facility with a history of violations, doctors and pharmacists need to know.
It’s not about bringing everything home. It’s about building a system that can withstand shocks. One that doesn’t collapse when a single factory has a bad inspection or a tornado hits.
Right now, we’re treating generic drug shortages like accidents. They’re not. They’re the result of deliberate choices-about pricing, regulation, and global trade. And those choices are costing patients their health.
Why are generic drug shortages getting worse?
Generic drug shortages are worsening because of low profit margins, extreme reliance on a few overseas manufacturers, and consolidation of production to just one or two suppliers. When one facility shuts down-due to quality issues, natural disasters, or regulatory action-there’s no backup. The system was designed for low cost, not resilience.
Are brand-name drugs affected by shortages too?
Rarely. Brand-name manufacturers have higher profit margins, diversified supply chains, and often keep large inventory buffers. They can absorb disruptions. Generic manufacturers operate on razor-thin margins and rely on single-source suppliers, making them far more vulnerable.
Can tariffs solve the generic drug shortage problem?
No. Tariffs would raise the cost of imported APIs and finished drugs, making already low-margin generics even less profitable. This could push more manufacturers out of the market, reduce competition, and worsen shortages. Experts warn tariffs could trigger higher prices and delayed treatments without fixing the root cause.
What drugs are most likely to be in short supply?
Sterile injectables-like IV fluids, antibiotics, chemotherapy drugs (cisplatin, doxorubicin), epinephrine, and heparin-are the most vulnerable. They’re complex to produce, have short shelf lives, and are often made by only one or two manufacturers. Older, low-cost generics are also at high risk.
How do drug shortages affect hospitals and pharmacists?
Hospitals spend hours each week finding alternatives, compounding drugs, or rationing supplies. Pharmacists may spend 20-30% of their time managing shortages instead of patient care. Clinicians are forced to use less effective or riskier substitutes, which can lead to worse outcomes. Some surgeries and treatments are delayed or canceled entirely.
Is there a solution being proposed that might actually work?
Yes. Proposals like S.2062, which would require manufacturers to maintain six-month reserves of critical generics, could help. So would government-backed minimum pricing for essential drugs, incentives for new manufacturers to enter the market, and mandatory transparency about where active ingredients are sourced. These solutions focus on fixing the economics, not just the geography.





10 comments
Kayleigh Campbell
They call it a supply chain issue but it’s really a profit-over-life issue. We’ve turned life-saving meds into commodities you bid on like used lawn mowers. When the cheapest bidder wins, someone’s gonna lose - and it’s always the patient.
Randolph Rickman
My cousin’s a hospital pharmacist in Ohio. She told me last week she spent 4 hours just tracking down saline bags. Four hours. That’s four hours she didn’t spend talking to patients, answering questions, or making sure someone got their meds right. This isn’t a policy problem - it’s a moral failure.
Colleen Bigelow
China and India are running our medicine supply like a Ponzi scheme. We let foreign countries control our health because we’re too lazy to make things here. Now we’re paying with lives. Time to shut down the imports and build factories - not just for drugs, but for American jobs and security. Tariffs? Bring ‘em on. Better than burying our grandparents because we couldn’t afford to make epinephrine.
SHAMSHEER SHAIKH
As someone from India, I must say - this is not about blame. Our factories are working under impossible conditions: low prices, high demand, and zero support. We are not villains - we are the last line of defense for millions who cannot afford brand-name drugs. The solution is not to cut us off, but to invest in us - fair pricing, better oversight, shared technology. Let’s fix the system, not the scapegoat.
Andrew Sychev
So what? People have been dying from bad meds since the 1950s. This isn’t new. The system is broken. The politicians are corrupt. The manufacturers are greedy. The FDA is asleep. And you? You’re still reading this instead of calling your rep. Wake up. Or don’t. I don’t care anymore.
sue spark
My dad needed heparin last year. They gave him a substitute. He had a stroke. The doctor said it wasn’t the drug’s fault. But I know. I saw the paperwork. The FDA label said ‘Made in China’ and nothing else. Why don’t we know this before we take it? Why is this secret?
Billy Poling
It is imperative to recognize that the structural deficiencies in the current pharmaceutical distribution model are not merely logistical, but fundamentally economic in nature, and thus necessitate a comprehensive policy intervention that addresses the perverse incentives inherent in the current competitive bidding frameworks, which have systematically disincentivized quality assurance, redundancy, and long-term investment in manufacturing infrastructure, thereby creating a systemic vulnerability that manifests in predictable, recurring, and entirely preventable public health crises.
James Rayner
i just think about how many people are out there right now, waiting for a shot, a bag of fluids, a pill that’s supposed to keep them alive… and it’s not there. not because of war or disaster… but because no one wanted to pay $3 instead of $1.50. that’s the saddest part. 💔
Tiffany Machelski
my pharmacy ran out of metformin last month. i had to wait 11 days. i just want someone to fix this please
Aditya Kumar
lol