When you or a loved one needs a simple, life-saving generic drug-like an antibiotic, an insulin injection, or a chemotherapy agent-and the pharmacy says it’s out of stock, it’s not just bad luck. It’s the result of a broken system. Generic drugs make up 90% of all prescriptions filled in the U.S., yet they account for 95% of all drug shortages. Why? The answer isn’t one thing. It’s a chain of failures: poor manufacturing, fragile supply lines, and a market that punishes the very drugs we rely on most.
Manufacturing Problems Are the #1 Cause
More than half of all drug shortages-62% according to FDA data from 2020-come down to one thing: manufacturing failures. These aren’t minor hiccups. They’re full shutdowns. A single batch of contaminated injectable medication can shut down a plant for months. Equipment breaks down. Clean rooms fail. Quality inspections turn up violations. And when that happens, production stops. No product. No backup. No quick fix.
Unlike branded drugs, which often have multiple factories making the same medicine, generic drugs are usually made in just one or two facilities. If one of them goes offline, there’s no one else to step in. This is called “sole sourcing.” One in five drug shortages happens because a single plant is the only source for that drug. And those plants? Many are aging. Some were built in the 1980s and never upgraded. Others were built overseas, where oversight is harder.
Most Active Ingredients Come From Just Two Countries
Think of a pill as a two-part system: the active ingredient (API) and the finished pill form. The API is the part that actually works. About 80% of all active pharmaceutical ingredients used in U.S. generic drugs are made in just two countries: China and India. That’s not diversity-it’s a single point of failure.
When a flood hits a factory in India, or a new regulation shuts down a facility in China, the ripple effect hits U.S. hospitals within weeks. In 2020, a lockdown in China delayed shipments of heparin, a blood thinner used in every ICU. In 2021, a fire at a major API plant in India caused shortages of dozens of antibiotics. These aren’t rare events. They’re predictable.
And here’s the kicker: the U.S. doesn’t have enough domestic manufacturing capacity to fill the gap. Less than half of finished drug manufacturing sites are in the U.S. And even those are running at near-maximum capacity. There’s no buffer. No spare room. No safety net.
No One Makes Extra Supply-Because It Doesn’t Pay
Why don’t manufacturers just build extra capacity? Because they can’t afford to. Generic drugs are sold at razor-thin margins. While branded drugs can make 30-40% profit, generics often make less than 15%. Some barely break even.
When a generic drug first hits the market, multiple companies rush in to make it. Prices drop fast. Within months, the cheapest bidder wins. Hospitals and pharmacies buy the lowest-cost option. That means manufacturers are forced to cut costs everywhere-labor, maintenance, quality control, even staffing. Many companies shut down older plants and consolidate production into fewer, cheaper facilities. That sounds smart on paper. In reality, it makes the system more fragile.
Over 3,000 generic products have been discontinued since 2010. Why? Because no one could make money on them anymore. Even when a drug is in shortage, manufacturers won’t restart production if they know they’ll be forced to sell it for pennies. The market doesn’t reward reliability-it rewards the lowest bid.
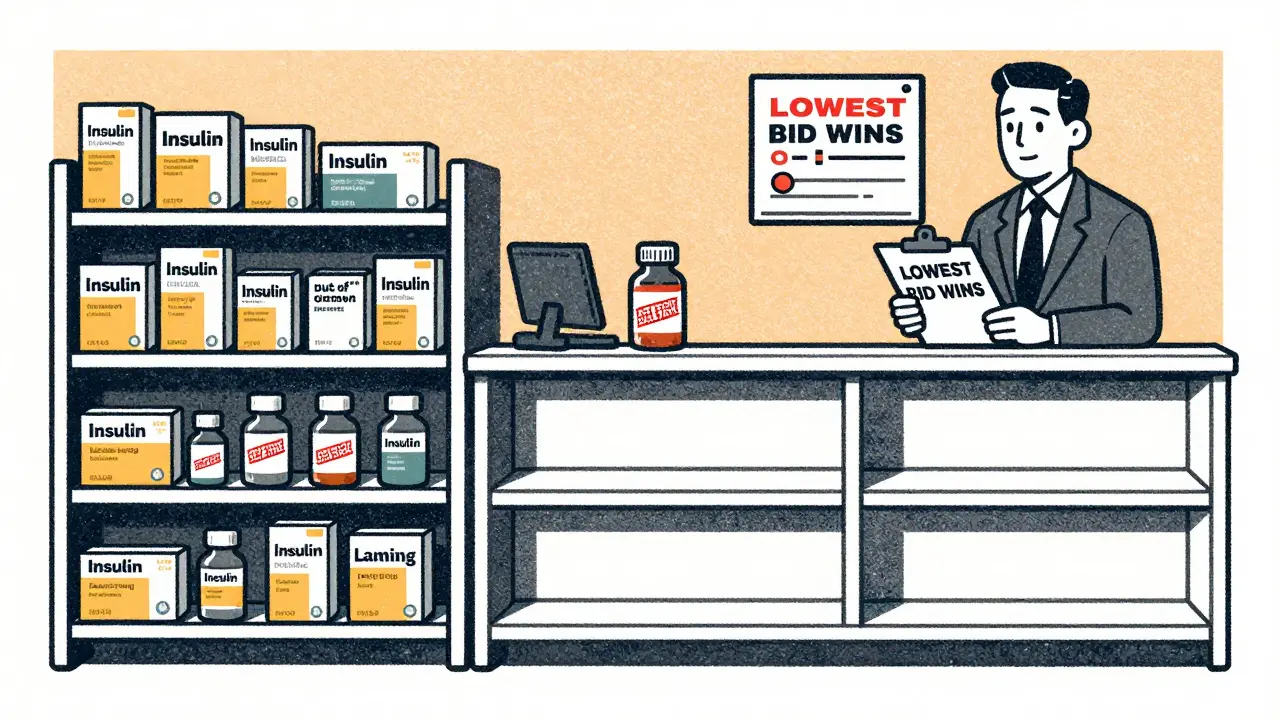
Pharmacy Benefit Managers Control the Market-and Make It Worse
Who decides which generic drug gets bought? It’s not the doctor. It’s not the hospital. It’s pharmacy benefit managers, or PBMs. These are middlemen who negotiate drug prices between manufacturers and insurers. Three PBMs control 85% of all prescription drug spending in the U.S.
They don’t just negotiate prices. They decide which drugs get placed on formularies-lists of approved medications. If a manufacturer offers the lowest price, their drug gets on the list. If another drug is slightly more expensive-even if it’s more reliable-it gets dropped. This creates a race to the bottom. Manufacturers slash prices to stay in the game. That means less money for quality control, less money for maintenance, less money to hire skilled workers.
The Federal Trade Commission found in 2023 that PBMs make these decisions without transparency. No public records. No accountability. And when a drug goes into shortage, PBMs don’t always switch to alternatives. Sometimes they keep pushing the same drug-even if it’s not available-because their contracts favor the cheapest option, not the most reliable one.
Canada Does It Better-Here’s How
Canada faces the same global supply chain risks. But their shortage rates are far lower. Why? Because they treat drug supply as a public health issue, not a market competition.
Canada has a national stockpile of critical generic drugs. When a shortage hits, they pull from it. They also have a centralized system where regulators, hospitals, manufacturers, and wholesalers talk to each other daily. If a plant in India shuts down, Canada doesn’t wait for a market signal. They call the manufacturer, find out when production will resume, and tell hospitals to adjust their orders.
In the U.S., no one has that kind of coordination. Hospitals scramble. Pharmacists spend 50-75% more time managing shortages than they did 10 years ago. Some don’t even know why a drug is out. One in four shortage reports in the U.S. have no explanation at all.

What’s Being Done-and Why It’s Not Enough
There are efforts to fix this. The RAPID Reserve Act, introduced in 2023, proposes building a strategic reserve of critical generic drugs. It also offers tax incentives to bring API manufacturing back to the U.S. It’s a step in the right direction. But it’s not a fix.
Real change requires two things: money and structure. We need to pay manufacturers enough to maintain quality and keep backup capacity. We need to stop letting PBMs choose drugs based only on price. We need to invest in domestic manufacturing-not just as a backup, but as a core part of the system.
Right now, the system is designed to be cheap, not resilient. But when your life depends on a $0.10 pill, cheap doesn’t work. Resilience does.
What This Means for Patients and Providers
Patients don’t just wait longer for their meds. They get switched to alternatives that might not work as well. A cancer patient might get a less effective chemo drug because the original is out. A newborn in the NICU might get a different antibiotic because the standard one isn’t available. These aren’t hypotheticals. They happen every week.
Hospital pharmacists are the frontline. They’re the ones calling suppliers, checking inventory, tracking expiration dates, and finding workarounds. They’re not trained to be supply chain managers. But they’re forced into that role because no one else is.
The system is broken because we treat generic drugs like commodities. But they’re not. They’re essential medicine. And when the supply chain cracks, people suffer.
Why are generic drugs more likely to be in shortage than brand-name drugs?
Generic drugs are more likely to be in shortage because they’re made by multiple companies competing on price, which drives profit margins so low that manufacturers can’t afford to maintain backup capacity or upgrade facilities. Brand-name drugs, by contrast, have patent protection that allows higher prices and more investment in production. Generic drugs also rely on a small number of manufacturing sites-often overseas-with no redundancy, while brand-name drugs typically have multiple production lines.
Do drug shortages affect all types of medications equally?
No. Sterile injectables-like antibiotics, anesthetics, and chemotherapy drugs-are the most vulnerable. These require complex, clean-room manufacturing, and even small contamination shuts down production. Oral pills are less likely to be affected because they’re easier to make and store. But even common oral generics like metformin or lisinopril have had shortages due to API supply issues from India and China.
Why don’t manufacturers just build more factories to prevent shortages?
Building new manufacturing facilities costs hundreds of millions of dollars and takes years to get approved. For generic drugs, the return on investment is too low. A company might spend $500 million to build a new plant, but if the drug sells for $0.05 per pill and competition drives prices down further, they’ll never recoup that cost. It’s a business decision: why risk it when you can make money on higher-margin drugs instead?
Can the U.S. rely on domestic manufacturing to solve this?
Not on its own. Even if all U.S. manufacturing capacity doubled, it wouldn’t cover the volume of generic drugs we use. The real solution is a mix: more domestic production for critical drugs, better international coordination, and strategic stockpiles. Relying only on one country-whether it’s the U.S., China, or India-leaves us vulnerable. Resilience requires redundancy.
What role do pharmacy benefit managers (PBMs) play in drug shortages?
PBMs control 85% of U.S. prescription drug spending and choose which drugs insurers cover based on price, not reliability. They often favor the cheapest generic-even if it’s from a single, unstable source-over a more reliable but slightly more expensive alternative. This creates a race to the bottom, where manufacturers cut corners to win bids, increasing the risk of shutdowns and shortages. PBMs don’t have to disclose how they make these decisions, making it hard to fix the problem.
What Needs to Change
Fixing this won’t be easy. But it’s not impossible. We need to stop pretending that a free market will solve a public health crisis. Here’s what needs to happen:
- Create a national stockpile of critical generic drugs, especially sterile injectables.
- Incentivize domestic manufacturing with tax breaks and guaranteed minimum purchase agreements for essential drugs.
- Require transparency from PBMs-make their formulary decisions public and subject to review.
- Build redundancy into the supply chain by requiring at least two approved manufacturers for every critical drug.
- Support international cooperation to monitor and respond to global supply disruptions.
The next time a pharmacy runs out of a generic drug, don’t assume it’s just bad luck. It’s a system failure. And it’s one we designed.






1 comments
Irish Council
This whole system is rigged. One factory in China shuts down and we're all screwed. And nobody talks about how the FDA lets these places slide for years. It's not coincidence. It's control.